Advertisement
Grab your lab coat. Let's get started
Welcome!
Welcome!
Create an account below to get 6 C&EN articles per month, receive newsletters and more - all free.
It seems this is your first time logging in online. Please enter the following information to continue.
As an ACS member you automatically get access to this site. All we need is few more details to create your reading experience.
Not you? Sign in with a different account.
Not you? Sign in with a different account.
ERROR 1
ERROR 1
ERROR 2
ERROR 2
ERROR 2
ERROR 2
ERROR 2
Password and Confirm password must match.
If you have an ACS member number, please enter it here so we can link this account to your membership. (optional)
ERROR 2
ACS values your privacy. By submitting your information, you are gaining access to C&EN and subscribing to our weekly newsletter. We use the information you provide to make your reading experience better, and we will never sell your data to third party members.
Analytical Chemistry
New Perspective on Reactions
Molecule's point of view reveals much about evolution of molecular dynamics
by Mitch Jacoby
December 19, 2005
| A version of this story appeared in
Volume 83, Issue 51

Spectroscopy
Hitching a ride on the back of a molecule can be tough. But if you could hang on over the course of a reaction, you'd see a chemical process unfolding from a unique perspective, the molecule's point of view.
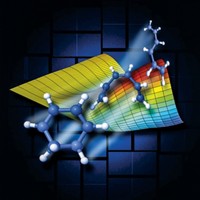
Researchers have now developed a technique that enables them to observe a chemical reaction from the molecular frame of reference. The study broadens understanding of chemical reaction dynamics and provides an up-close and detailed view of the evolution of chemical bonds and electronic states.
As exciting as it may be to watch sporting events from the stands or by way of a camera positioned in the photographers' pit, fans viewing from those vantage points miss a lot of the action. That's why broadcasters have worked out ways to provide a view from a tiny camera in the helmet of a downhill skier or automobile racer, for example.
It's much the same with chemistry. Ultrafast laser methods can monitor the motions of atoms during chemical reactions, but generally they do so from the laboratory reference frame. From that perspective, molecular information may be missed because molecules in gas-phase samples are constantly tumbling about and are present in every orientation.
Now, through a combination of time-resolved spectroscopy methods and quantum mechanical calculations, scientists have devised a procedure for monitoring a reaction pathway completely, from reactants to products, on the femtosecond (fs) timescale from the perspective of the molecule undergoing reaction. Applied to a test case, dissociation of the nitric oxide dimer (O=NN=O), the technique reveals that the dimer, which is excited with an ultraviolet laser pulse, starts off with its electron cloud in a form-hugging or valence state and evolves within about 150 fs to a more diffuse configuration known as a Rydberg state. About 600 fs later, the Rydberg species finally dissociates (Science, published online Dec. 15, dx.doi.org/10.1126/science.1120779).
The research team includes Albert Stolow, Oliver Gessner, and Anthony M. D. Lee of the Canadian National Research Council in Ottawa; Carl C. Hayden of Sandia National Laboratories, Livermore, Calif.; and their coworkers at the University of Southern California and elsewhere.
"You could not possibly get this information from the lab reference frame," Stolow asserts. He adds that studying either the absorption spectrum or the final products "provides no hint of this two-step dissociation process."
There's no question it's a fundamental study, Stolow says. "But we expect that investigating femtochemistry from the molecule's point of view will shed light on the dynamical evolution of increasingly complex chemical processes."

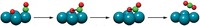

Join the conversation
Contact the reporter
Submit a Letter to the Editor for publication
Engage with us on Twitter