Advertisement
Grab your lab coat. Let's get started
Welcome!
Welcome!
Create an account below to get 6 C&EN articles per month, receive newsletters and more - all free.
It seems this is your first time logging in online. Please enter the following information to continue.
As an ACS member you automatically get access to this site. All we need is few more details to create your reading experience.
Not you? Sign in with a different account.
Not you? Sign in with a different account.
ERROR 1
ERROR 1
ERROR 2
ERROR 2
ERROR 2
ERROR 2
ERROR 2
Password and Confirm password must match.
If you have an ACS member number, please enter it here so we can link this account to your membership. (optional)
ERROR 2
ACS values your privacy. By submitting your information, you are gaining access to C&EN and subscribing to our weekly newsletter. We use the information you provide to make your reading experience better, and we will never sell your data to third party members.
Synthesis
NH3's synthetic appeal gets a boost
July 24, 2006
| A version of this story appeared in
Volume 84, Issue 30
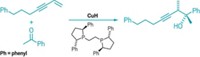
Ammonia is cheap and plentiful but rarely used as a reagent in catalytic processes. In hopes of broadening the synthetic appeal of this atom-economical nitrogen source, Yale University chemists have used NH3 in a catalytic process to make primary aromatic amines, an important class of chemical building blocks (J. Am. Chem. Soc., DOI: 10.1021/ja064005t). John F. Hartwig and Qilong Shen use NH3 and a palladium catalyst bearing the commercial Josiphos ligand (shown) to convert aryl halides into primary arylamines. Previous palladium-catalyzed syntheses of primary arylamines have relied on ammonia surrogates. The catalytic system Hartwig and Shen report affords good yields and excellent selectivity with NH3, but they will have to overcome a number of hurdles for the reaction to find commercial use, including lowering catalyst loads, reducing dilution requirements, and increasing functional group tolerance. That quest will be helped by further study of the reaction's mechanism, which involves a never-before-seen C-N bond-forming reductive elimination of an NH2 complex, the authors report.



Join the conversation
Contact the reporter
Submit a Letter to the Editor for publication
Engage with us on Twitter