Advertisement
Grab your lab coat. Let's get started
Welcome!
Welcome!
Create an account below to get 6 C&EN articles per month, receive newsletters and more - all free.
It seems this is your first time logging in online. Please enter the following information to continue.
As an ACS member you automatically get access to this site. All we need is few more details to create your reading experience.
Not you? Sign in with a different account.
Not you? Sign in with a different account.
ERROR 1
ERROR 1
ERROR 2
ERROR 2
ERROR 2
ERROR 2
ERROR 2
Password and Confirm password must match.
If you have an ACS member number, please enter it here so we can link this account to your membership. (optional)
ERROR 2
ACS values your privacy. By submitting your information, you are gaining access to C&EN and subscribing to our weekly newsletter. We use the information you provide to make your reading experience better, and we will never sell your data to third party members.
Synthesis
Concurrent N-H, C-H Bond Activations
November 27, 2006
| A version of this story appeared in
Volume 84, Issue 48
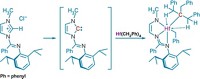
Some metal complexes can activate hydrocarbon C-H bonds intermolecularly and some can activate N-H bonds, but now chemists have discovered that a family of allyl nitrosyl complexes of tungsten can concurrently activate both the N-H and α-C-H bonds of pyrrolidine or piperidine (J. Am. Chem. Soc. 2006, 128, 14762). For example, when the complex shown (top) reacts with neat pyrrolidine at room temperature, the resulting complex (bottom) sports a pyrrolide ligand; the allyl ligand of the starting complex has become a vinylalkyl substituent of the new pyrrolide ligand. The hydrogen atoms on the pyrrolidine's nitrogen and α-carbon atoms are eliminated as H2. Peter Legzdins of the University of British Columbia, Vancouver, whose group made the discovery, believes this type of transformation is unprecedented.



Join the conversation
Contact the reporter
Submit a Letter to the Editor for publication
Engage with us on Twitter