Advertisement
Grab your lab coat. Let's get started
Welcome!
Welcome!
Create an account below to get 6 C&EN articles per month, receive newsletters and more - all free.
It seems this is your first time logging in online. Please enter the following information to continue.
As an ACS member you automatically get access to this site. All we need is few more details to create your reading experience.
Not you? Sign in with a different account.
Not you? Sign in with a different account.
ERROR 1
ERROR 1
ERROR 2
ERROR 2
ERROR 2
ERROR 2
ERROR 2
Password and Confirm password must match.
If you have an ACS member number, please enter it here so we can link this account to your membership. (optional)
ERROR 2
ACS values your privacy. By submitting your information, you are gaining access to C&EN and subscribing to our weekly newsletter. We use the information you provide to make your reading experience better, and we will never sell your data to third party members.
Synthesis
New fluorescein adds to cellular probe arsenal
February 20, 2006
| A version of this story appeared in
Volume 84, Issue 8
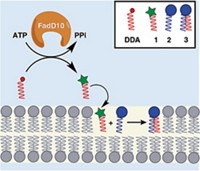
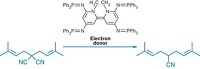
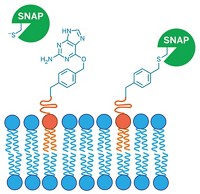
Pennsylvania Green is a newly synthesized fluorescein derivative reported to improve the pH range and photostability of the classical green fluorophore (Org. Lett. 2006, 8, 581). Switching key fluorescein substituents has already proven useful to optimize fluorescein to function under a broader range of pH conditions and light intensity. For example, commercially available Oregon Green has two fluorine substituents on the molecule's xanthene core, improving fluorescence at lower pH. Tokyo Green replaces the carboxylate group with a methyl group, increasing hydrophobicity to facilitate monitoring of intracellular activity. Pennsylvania Green, synthesized by Laurie F. Mottram in Blake R. Peterson's research group at Pennsylvania State University, is a hybrid of Oregon Green and Tokyo Green, incorporating both the fluorine and methyl substituents and reaping the benefits of both probes. Pennsylvania Green is less polar than Oregon Green and has better photostability than Tokyo Green, making it a potentially ideal probe for investigating cellular biology, Peterson says.



Join the conversation
Contact the reporter
Submit a Letter to the Editor for publication
Engage with us on Twitter