Advertisement
Grab your lab coat. Let's get started
Welcome!
Welcome!
Create an account below to get 6 C&EN articles per month, receive newsletters and more - all free.
It seems this is your first time logging in online. Please enter the following information to continue.
As an ACS member you automatically get access to this site. All we need is few more details to create your reading experience.
Not you? Sign in with a different account.
Not you? Sign in with a different account.
ERROR 1
ERROR 1
ERROR 2
ERROR 2
ERROR 2
ERROR 2
ERROR 2
Password and Confirm password must match.
If you have an ACS member number, please enter it here so we can link this account to your membership. (optional)
ERROR 2
ACS values your privacy. By submitting your information, you are gaining access to C&EN and subscribing to our weekly newsletter. We use the information you provide to make your reading experience better, and we will never sell your data to third party members.
Environment
Sulfur-cutting enzyme up close
February 20, 2006
| A version of this story appeared in
Volume 84, Issue 8
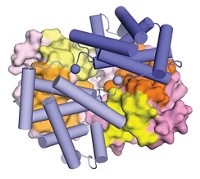
The primordial linkage between geology and life meets headon in sulfur-oxidizing enzymes within ancient microbes such as Acidianus ambivalens, which thrive in volcanic hot springs at 80 °C and pH 2. With a new X-ray crystallographic structure of this archaeon's sulfur oxygenase reductase, Carlos Frazão of the New University of Lisbon, in Portugal, and his colleagues have uncovered key details about how the enzyme processes elemental sulfur into bisulfite ion and hydrogen sulfide under aerobic conditions (Science 2006, 311, 996). "This enzyme bridges the geo- and biological global sulfur cycles," Frazão notes. When the enzyme's 24 protein monomers assemble, they form a sphere with a hollow starlike compartment (cross section shown), which hosts two dozen catalytic pockets. The key features of each are a cysteine molecule with an extra sulfur atom bound to it and a nearby iron center. Besides helping to explain the global sulfur cycle, the structural data should help scientists discern how certain microbes extract metals from sulfidic ores and produce environmentally troublesome acidic effluent in mines.




Join the conversation
Contact the reporter
Submit a Letter to the Editor for publication
Engage with us on Twitter