Advertisement
Grab your lab coat. Let's get started
Welcome!
Welcome!
Create an account below to get 6 C&EN articles per month, receive newsletters and more - all free.
It seems this is your first time logging in online. Please enter the following information to continue.
As an ACS member you automatically get access to this site. All we need is few more details to create your reading experience.
Not you? Sign in with a different account.
Not you? Sign in with a different account.
ERROR 1
ERROR 1
ERROR 2
ERROR 2
ERROR 2
ERROR 2
ERROR 2
Password and Confirm password must match.
If you have an ACS member number, please enter it here so we can link this account to your membership. (optional)
ERROR 2
ACS values your privacy. By submitting your information, you are gaining access to C&EN and subscribing to our weekly newsletter. We use the information you provide to make your reading experience better, and we will never sell your data to third party members.
Pharmaceuticals
New Way to Target HIV
Novel drug-binding site found on HIV reverse transcriptase
by Stu Borman
January 1, 2007
| A version of this story appeared in
Volume 85, Issue 1

A newly revealed drug-binding site on human immunodeficiency virus reverse transcriptase could lead to a new class of HIV medications.
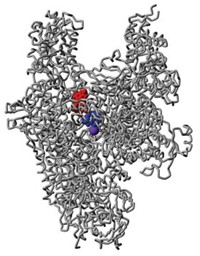
The site was observed in the X-ray crystal structure of a complex of HIV reverse transcriptase with the inhibitor dihydroxybenzoyl naphthyl hydrazone (DHBNH). The structure was determined and analyzed by Daniel M. Himmel and Stefan G. Sarafianos in structural biologist Eddy Arnold's group at the Center for Advanced Biotechnology & Medicine, Rutgers University, and their coworkers (ACS Chem. Biol. 2006, 1, 702).
Structures of drug complexes of HIV reverse transcriptase have been obtained before, but the site at which DHBNH binds was not observed in any of those.
Reverse transcriptase has both RNase H (RNA-cleaving) and DNA polymerase (DNA-synthesizing) activity. Both activities are needed to reverse-transcribe HIV's RNA-based genomic material into DNA, an essential part of the virus's life cycle.
DHBNH primarily inhibits the enzyme's RNase H activity. So Arnold and coworkers were surprised to find that the inhibitor binds at a spot near the DNA polymerase domain. In their paper, they propose mechanisms for the drug's remote effect.
"This binding site has never before been described and could conceivably become the target for a new class of anti-HIV drugs," Arnold says. The binding site is very close to that of nonnucleoside reverse transcriptase inhibitors such as efavirenz (Sustiva). But those drugs inhibit the enzyme's DNA polymerase activity primarily, not its RNase H function.
DHBNH is not a viable drug candidate. But the new structure could aid the design of agents that inhibit the RNase H activity more potently or inhibit both of the enzyme's activities. In the paper, coauthor Michael A. Parniak of the University of Pittsburgh and coworkers report having synthesized compounds that do inhibit both activities.
Most HIV drugs that are approved or in clinical trials work by inhibiting the virus's protease or integrase enzymes or the DNA polymerase activity of reverse transcriptase. Reverse transcriptase's RNase H activity will be next as a drug target, Arnold predicts, and the new structure "is an important step in that direction," he says.
John M. Coffin, an HIV expert at Tufts University, says, "The oddity of the new study is that the drug doesn't bind to the place you expect. That leads to the possibility that you can create an inhibitor that blocks both active sites at once, which Arnold and coworkers seem to have been able to do."



Join the conversation
Contact the reporter
Submit a Letter to the Editor for publication
Engage with us on Twitter