Advertisement
Grab your lab coat. Let's get started
Welcome!
Welcome!
Create an account below to get 6 C&EN articles per month, receive newsletters and more - all free.
It seems this is your first time logging in online. Please enter the following information to continue.
As an ACS member you automatically get access to this site. All we need is few more details to create your reading experience.
Not you? Sign in with a different account.
Not you? Sign in with a different account.
ERROR 1
ERROR 1
ERROR 2
ERROR 2
ERROR 2
ERROR 2
ERROR 2
Password and Confirm password must match.
If you have an ACS member number, please enter it here so we can link this account to your membership. (optional)
ERROR 2
ACS values your privacy. By submitting your information, you are gaining access to C&EN and subscribing to our weekly newsletter. We use the information you provide to make your reading experience better, and we will never sell your data to third party members.
Biological Chemistry
Shape-Shifting Antibiotic Resistance
In an unprecedented mechanism, MRSA’s protein mutants are found to disrupt allosteric changes that leave the bacterium vulnerable to antibiotics
by Elizabeth K. Wilson
July 7, 2014
| A version of this story appeared in
Volume 92, Issue 27
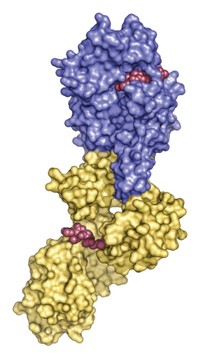
The antibiotic ceftaroline was approved by FDA in late 2010 to battle the problematic methicillin-resistant Staphylococcus aureus (MRSA) bacterium. But studies have shown that the ever-mutating organism has already developed resistance to this antibiotic. A team led by Shahriar Mobashery of the University of Notre Dame and Juan A. Hermoso of the Spanish National Research Council now shows that a hitherto unrecognized mechanism—the prevention of allosteric motions that allow the antibiotic to bind to a bacterial protein—is responsible for this resistance (J. Am. Chem. Soc. 2014, DOI: 10.1021/ja5030657). β-Lactam antibiotics such as penicillins and cephalosporins kill bacteria by disrupting cell-wall biosynthesis. However, mutations in the active site of the target protein prevent the antibiotic from binding. Ceftaroline’s action involves two steps: First, a ceftaroline molecule causes an allosteric change in the target protein; then this change makes the protein’s active site available for binding by a second molecule. With kinetic studies and X-ray crystallography, the researchers show that mutations in the MRSA protein now prevent the first ceftaroline molecule from causing those crucial conformational changes. Because of the importance of allostery in many biological systems, the authors argue that such allosteric mechanisms may also be at work in other antibiotic-resistant systems.


Join the conversation
Contact the reporter
Submit a Letter to the Editor for publication
Engage with us on Twitter