Advertisement
Grab your lab coat. Let's get started
Welcome!
Welcome!
Create an account below to get 6 C&EN articles per month, receive newsletters and more - all free.
It seems this is your first time logging in online. Please enter the following information to continue.
As an ACS member you automatically get access to this site. All we need is few more details to create your reading experience.
Not you? Sign in with a different account.
Not you? Sign in with a different account.
ERROR 1
ERROR 1
ERROR 2
ERROR 2
ERROR 2
ERROR 2
ERROR 2
Password and Confirm password must match.
If you have an ACS member number, please enter it here so we can link this account to your membership. (optional)
ERROR 2
ACS values your privacy. By submitting your information, you are gaining access to C&EN and subscribing to our weekly newsletter. We use the information you provide to make your reading experience better, and we will never sell your data to third party members.
Synthesis
Sorting Nanotube Isomers
May 14, 2007
| A version of this story appeared in
Volume 85, Issue 20
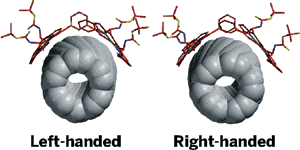
Optical isomers of single-walled carbon nanotubes (SWNTs) now can be sorted from one another for the first time, thanks to a pair of chiral diporphyrin "tweezers" developed by researchers in Japan (Nat. Nanotechnol., DOI: 10.1038/nnano.2007.142). Commercially available SWNTs usually are a jumble of nanotubes of various lengths and diameters, and this lack of uniformity can make product development difficult. Nanotubes also possess a helical twist-either right- or left-handed-that gives them optical activity. Using one or the other of the chiral diporphyrin molecules, a group led by Xiaobin Peng and Naoki Komatsu of Shiga University of Medical Science was able to separate the optical isomers. The sorting process takes advantage of the diporphyrins' affinity to bind more tightly to one of the two helices (one diporphyrin shown). The researchers suspended the SWNTs and a chiral diporphyrin in methanol, followed by sonication and centrifugation. The resulting supernatant liquid is enriched with the preferred diporphyrin-SWNT complex. The diporphyrin is easily washed away, leaving behind the SWNT isomer.


Join the conversation
Contact the reporter
Submit a Letter to the Editor for publication
Engage with us on Twitter