Advertisement
Grab your lab coat. Let's get started
Welcome!
Welcome!
Create an account below to get 6 C&EN articles per month, receive newsletters and more - all free.
It seems this is your first time logging in online. Please enter the following information to continue.
As an ACS member you automatically get access to this site. All we need is few more details to create your reading experience.
Not you? Sign in with a different account.
Not you? Sign in with a different account.
ERROR 1
ERROR 1
ERROR 2
ERROR 2
ERROR 2
ERROR 2
ERROR 2
Password and Confirm password must match.
If you have an ACS member number, please enter it here so we can link this account to your membership. (optional)
ERROR 2
ACS values your privacy. By submitting your information, you are gaining access to C&EN and subscribing to our weekly newsletter. We use the information you provide to make your reading experience better, and we will never sell your data to third party members.
Policy
Warnings issued on diabetes drugs
June 11, 2007
| A version of this story appeared in
Volume 85, Issue 24

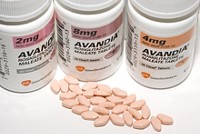
On June 6, FDA revealed it has issued strict warnings of heart risks on two type 2 diabetes drugs, Avandia and Actos, which are in the same therapeutic class. At a May 23 hearing of the House Committee on Oversight & Government Reform, FDA Commissioner Andrew C. von Eschenbach said he had asked the manufacturers of the drugs to place the most prominent type of warning on the labels, a "black box warning." But the request was not made public until the hearing. Von Eschenbach stressed that studies on the association between Avandia (rosiglitazone maleate) and heart risks are inconsistent and that the dangers did not become clear until recently. A comprehensive reanalysis of the data performed at FDA this year and a study published on May 21 in the New England Journal of Medicine confirmed the possibility that Avandia may pose excess risks, he said. However, Rep. Henry A. Waxman (D-Calif.) said FDA was remiss in not requiring the drug's manufacturer GlaxoSmithKline "to conduct a thorough postmarket study of Avandia's heart risks."


Join the conversation
Contact the reporter
Submit a Letter to the Editor for publication
Engage with us on Twitter