Advertisement
Grab your lab coat. Let's get started
Welcome!
Welcome!
Create an account below to get 6 C&EN articles per month, receive newsletters and more - all free.
It seems this is your first time logging in online. Please enter the following information to continue.
As an ACS member you automatically get access to this site. All we need is few more details to create your reading experience.
Not you? Sign in with a different account.
Not you? Sign in with a different account.
ERROR 1
ERROR 1
ERROR 2
ERROR 2
ERROR 2
ERROR 2
ERROR 2
Password and Confirm password must match.
If you have an ACS member number, please enter it here so we can link this account to your membership. (optional)
ERROR 2
ACS values your privacy. By submitting your information, you are gaining access to C&EN and subscribing to our weekly newsletter. We use the information you provide to make your reading experience better, and we will never sell your data to third party members.
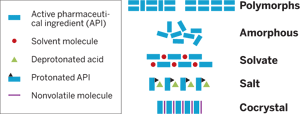
Pharmaceuticals
Form And Function
The choice of pharmaceutical crystalline form can be used to optimize drug properties, and cocrystals are emerging as new alternatives
by Ann M. Thayer
June 18, 2007
| A version of this story appeared in
Volume 85, Issue 25

"TAKE TWO ASPIRIN and call me in the morning," the medical adage goes. To most people, this means simply washing down two tablets. Until late 2005, "take two aspirin" didn't have any different connotation for crystallographic researchers either; as far as anyone knew, after 40 years of study, only one crystalline phase, or polymorph, of acetylsalicylic acid had been found.
Then a reported second polymorph unleashed a rancorous dispute over the subtleties of how molecules pack in crystals (C&EN, Jan. 1, page 27). Although the discovery isn't expected to have practical ramifications, it underscored the importance of identifying, making, and controlling the crystal forms of pharmaceuticals. A drug's behavior depends on much more than the molecular structure; its solid form dictates its properties, including stability, hygroscopicity, dissolution rate, solubility, and bioavailability.
COVER STORY
Form And Function
Pharmaceutical developers usually search for a crystal form with the best properties for therapeutic use and manufacturability. As a material, the selected form must be amenable to handling and processing, and as a drug, it must be effective and safe. One problem is that the most stable polymorph is also the least soluble. In addition, one can't know beforehand exactly how many polymorphs exist and under what conditions they'll appear.
The industry took note of this in 1998 when Abbott Laboratories had to halt sales of its HIV protease inhibitor, Norvir. It had commercialized a metastable form of ritanovir, the active pharmaceutical ingredient (API), in solution-filled gelcaps. When a more stable, previously unknown polymorph suddenly crystallized, the company had to reformulate the drug, which cost it an estimated $250 million in lost sales.
Meanwhile, the U.S. Food & Drug Administration became concerned about the effect that different solid forms could have on API quality and performance. Consequently, international regulators now require manufacturers to screen for polymorphs. When it comes to getting approval, beyond the usual safety and efficacy considerations, drugmakers may have to prove they understand the form they've chosen and can reliably control its production and stability.
Besides single-component polymorphs, drugs can also exist as salts and other multicomponent crystalline phases. For example, solvates and hydrates may contain an API host and either solvent or water molecules, respectively, as guests. Analogously, when the guest compound is a solid at room temperature, the resulting form is often called a cocrystal. Salts, solvates, hydrates, and cocrystals may show polymorphism as well.
According to a recent analysis by SSCI, the solid-state chemistry business of Aptuit, polymorph screens of 245 compounds revealed that about 90% of them exhibit multiple solid forms. These data are consistent with those from other sources. Overall, half the compounds were polymorphic, often having one to three forms. About one-third of the compounds formed hydrates, and about one-third formed solvates. Data from cocrystal screens of 64 compounds showed that 60% formed cocrystals other than hydrates or solvates.
Faced with so much variety, pharmaceutical companies choose their solid forms carefully. "We're interested in getting the right properties for a compound to be a good medicine," says Simon Black, crystallization scientist in process R&D at AstraZeneca.
"We always prefer to develop the most thermodynamically stable form, provided its other properties are acceptable," he says. If, however, a compound is insoluble, hygroscopic, or difficult to crystallize, they'll consider making salts or possibly cocrystals.
Apart from the regulatory obligations, polymorph screening is common practice to avoid surprises later in development, Black notes. Drug developers also want to identify and characterize as many forms of their proprietary compounds as possible. Beyond offering choices for optimal physical properties, each form may be patentable.
Drug companies usually file patents on all the different forms during development. Thus, when initial patents on the compound itself expire, they can conceivably extend a product's life by moving to another form. In turn, generic drugmakers will target unprotected forms to avoid patent infringement. Nevertheless, high-profile lawsuits around GlaxoSmithKline's Zantac and Paxil and Bristol-Myers Squibb's cefadroxil have hinged on solid-form issues.
Mounting litigation, Abbott's Norvir experience, regulatory changes, and other factors have all converged within the past decade. They have elevated concerns about solid forms and the desire to explore alternatives to salts and polymorphs. Although researchers have studied crystalline molecular complexes for more than 100 years, drug developers have only intermittently exploited cocrystal forms of pharmaceuticals.
In the past, there had been a lack of experience making cocrystals with APIs, along with a limited understanding of how cocrystals can change drug properties. No drug that can be definitively described as a cocrystal and not a salt has been approved, those working in the field say. But greater interest is emerging as both understanding and experience grow, and the need for new and better-performing solid forms increases. Cocrystals are potentially attractive for improving properties while leaving an API unaltered.
In a Molecular Pharmaceutics special section this month on pharmaceutical cocrystals, Andrew V. Trask, a legal intern at Jones Day law firm in New York City, lays out arguments he and others believe support why pharmaceutical cocrystals will continue to be an active area of scientific investigation. He also describes how they might offer commercial and legal opportunities and advantages over other solid forms (Mol. Pharmaceutics 2007, 4, 301).
As a former scientist at Pfizer, Trask himself is a co-inventor on patents related to crystal forms of APIs. After receiving his Ph.D. in chemistry in 2006 from Cambridge University, where he worked with chemistry professor Willam Jones, he now attends law school and is registered to practice before the U.S. Patent & Trademark Office (PTO).
"There's been a lot of legal activity around salts and polymorphs, but not yet for pharmaceutical cocrystals," Trask tells C&EN. Although there is a growing number of patent applications on cocrystals, PTO has yet to issue many patents. But similar forms, such as salts, hydrates, and solvates, have proven to be patentable.
TO BE PATENTABLE, a new crystal form must have utility, which is not usually an obstacle for most APIs. It must also be nonobvious and novel—that is, not previously appearing in the prior art. Fortunately for pharmaceutical cocrystals, the prior art is limited, says Eyal Barash, a chemist and Aptuit's chief patent counsel.
Prior art can be tricky, Barash explains, because it needs to only "inherently anticipate" a form's existence. For example, a patented method to make an anhydrate may also yield trace amounts of a hydrated form; even if the hydrate is never identified, it's still considered to have appeared in the prior art because the method makes it.
This catch has been the basis of litigation around polymorphs, hydrates, and solvates. "Cocrystals offer a better shot at surviving such an attack," Barash says, "because if you reproduce a method, and a component is not included, then you're never going to have made a cocrystal of the API with a component that's not there."
Crystallizing a salt may be attempted if an ionizable group is present. And the drug industry has a lot of experience screening for and making salts; according to Trask, more than 24,000 U.S. patents contain the term "pharmaceutically acceptable salt." But salt and cocrystal forms are not predictable, Trask and Barash say.
Salts of APIs are limited to acceptable counterions, but cocrystals can potentially be made using a broader range of guests or cocrystal formers. Although you can choose logical combinations to start with, you can't know in advance whether a salt or cocrystal will form, and it's impossible to predict the structure and its properties, Trask and Barash explain. Thus, researchers may resort to even more extensive screening to discover cocrystals.
"Cocrystals have the same advantages that polymorphs do from an obviousness perspective in being less predictable," Barash says. He recommends supporting any solid-form claim with high-quality, reproducible data drawn often from multiple analytical techniques.
This may be good for the overall patentability of cocrystals, but it means coverage will probably be narrow rather than over broad classes of compounds. "If you find and claim a really good cocrystal, that won't protect you against someone else claiming a different one," Barash says. "You'd have to do more experimental work to find as many cocrystals as you can and then protect each of them."
Others take a different view. Michael J. Zaworotko, chemistry department chairman at the University of South Florida, says he and collaborators at Johnson & Johnson's TransForm Pharmaceuticals are awaiting decisions on patent applications covering cocrystal classes. "Once you have established that you can make cocrystals of a particular drug with a particular type of cocrystal former, you should be able to claim a series," Zaworotko says.
"It's long established that you can claim a series of salts before they have been made," he says. "There's little rhyme or reason to why polymorphs or solvates form, and until you've made one, you really can't predict whether the crystal morphology, dissolution profile, and stability will be superior.
"With cocrystals, there's a bit more crystal engineering or design possible," he continues. "Before our work came along, it wasn't obvious that drugs would form cocrystals and that the cocrystals would have superior properties." In a 2004 article, Zaworotko and TransForm's Örn Almarsson highlighted the potential of pharmaceutical cocrystals (Chem. Commun. 2004, 1889).
Cocrystals have sparked the interest of many researchers in the crystal engineering field as a way to tailor material properties. Intermolecular interactions between neutral molecules hold these crystals together in well-defined and ordered crystalline arrays. To assemble cocrystals, scientists have been exploring molecular recognition events.
One place Zaworotko looks for these events is among the more than 130,000 organic crystal structures in the Cambridge Structural Database. Fewer than 1,500 are cocrystals held together by hydrogen bonding (J. Pharm. Sci. 2006, 95, 499). By evaluating the 1,500 structures, chemists can determine the occurrence and geometries of "supramolecular heterosynthons," or intermolecular pairings, which can be starting points for cocrystal design.
"Carboxylic acids, alcohols, and amides are quite prevalent in drugs," Zaworotko points out, "so you choose a cocrystal former that has better hydrogen-bonding complementarity with the drug than the drug with itself." The assumption is that a cocrystal will assemble if a favorable supramolecular heterosynthon forms.
But which heterosynthon pairing-for example, hydroxyl-pyridine, hydroxyl-amine, and hydroxyl-cyano-prevails among competing options can be determined only by characterizing the resulting cocrystals. Zaworotko and coworkers recently delineated such a hierarchy with a series of 17 cocrystals (Mol. Pharmaceutics 2007, 4, 401).

SIMILAR WORK on heterosynthons is under way in other research groups. At the University of Hyderabad, in India, Ashwini Nangia and collaborators have explored temperature effects and the reactivity of hydrogen-bonding groups in aromatic carboxylic acid and carboxamide cocrystals (Chem.-Asian J. 2007, 2, 505). Further work of theirs has looked specifically at cocrystal preparation with model APIs such as carbamazepine and barbituric acid that involve amide-N-oxide heterosynthons (Mol. Pharmaceutics 2007, 4, 417).
Interest in multicomponent crystals is actually decades old, with many research groups having laid the groundwork. The field's research portfolio spans studies focused on intermolecular interactions and crystal structures to applications in specialty chemicals, advanced materials, and, more recently, APIs. Still, the field of supramolecular chemistry is quite young compared with traditional organic or covalent chemistry.
Christer B. Aakeröy, a professor of chemistry at Kansas State University, sees parallels to the historical development of organic reactions and synthetic methods. "We are trying to replicate that and come up with a reliable synthetic paradigm for noncovalent synthesis," he says. "But it's not going to happen overnight or have the same kind of reliability, because we are dealing with weaker and reversible interactions.
Advertisement
"We are still looking to develop a drawer full of supramolecular reactions that we can pull out every time we see a molecule with certain functionalities," he adds. Eventually, he believes the odds can be stacked in the chemist's favor to give good supramolecular yields by better understanding which functional groups to bring together to form cocrystals with the desired connectivity.
To this end, Aakeröy's research group has undertaken systematic cocrystallization studies of hydrogen-bond-based synthons (CrystEngComm 2005, 7, 439 and 2007, 9, 46). A wide variety of synthons is needed to determine which ones can operate side by side without interfering with each other, thus providing the foundation for modular supramolecular synthesis based upon hierarchies of intermolecular interactions, he explains.
To further increase the options and chances for success, Aakeröy's group often covalently modifies the supramolecular reagents. Such synthetic changes can adjust the strength of hydrogen-bond donors or acceptors (CrystEngComm 2006, 8, 586; Chem. Commun. 2006, 1445) to fine-tune the compatibility between functional groups on the cocrystal former and an API.
Although most efforts at making cocrystals center on two components, there is no reason that multiple sites on an API can't be targeted. Aakeröy and coworkers have made ternary cocrystals of carboxylic acids with isonicotinamide (Angew. Chem. Int. Ed. 2001, 40, 3240) and with tailor-made pyridyl/benzimidazol-1-yl-based reagents (Chem. Commun. 2005, 2820).
Likewise, Nangia has made carboxylic acid-bipyridine ternary systems (Cryst. Growth Des. 2005, 5, 1683). And Joel Bernstein at Ben-Gurion University, in Israel, has explored a hydrogen-bonding synthon that could potentially involve up to four different molecules. He and graduate student Mazal Wenger attempted to make this synthon in cocrystals of γ-aminobutyric acid with oxalic or benzoic acid (Angew. Chem. Int. Ed. 2006, 45, 7966).
Because the number of possible combinations can quickly get out of hand, researchers believe systematic approaches are crucial when designing and making cocrystals. Drug company researchers often look for potential cocrystal formers among the hundreds of compounds on FDA's Generally Recognized as Safe (GRAS) list because they anticipate regulators may find these more acceptable in drug forms.
Others, such as Zaworotko, believe the list is creating "a certain amount of tunnel vision." His research group doesn't focus on using GRAS list compounds, but does choose cocrystal formers on the basis of melting point, cost, and toxicity.
From a chemistry point of view, Aakeröy believes there won't be much headway made by staying with compounds on the GRAS list. "The pharmaceutical industry is particularly keen to do that," he says, "but there is no reason to believe that those compounds would be perfect for interacting in a targeted way with complicated APIs."
Taking a different approach, Mino R. Caira, professor of chemistry at the University of Cape Town, in South Africa, has studied the possibility of using "old" drugs as cocrystal formers. In a recent paper, Caira reviews the cocrystallization of antimicrobial sulfonamide drugs with drugs in other classes (Mol. Pharmaceutics 2007, 4, 310).
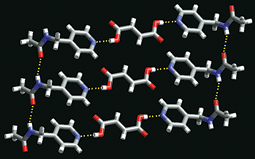
ALONG WITH attempts to design cocrystals, other researchers are investigating methods to synthesize and screen them. Cambridge University's Jones is among those exploring making cocrystals by simply grinding components together.
His group has extended the method by adding tiny, nonstoichiometric amounts of solvent that can enhance the rate and selectivity of cocrystal formation. With liquid-assisted grinding, they have even made ternary systems and found they could make more by grinding than from solution crystallization (Angew. Chem. Int. Ed. 2006, 45, 7546).
Grinding avoids the need to have all the components soluble in the same solvent. "In the vast majority of cases, we can get the same product by grinding as you can from solution," Jones says. "It's not that grinding is better, but it's a quick way of getting information." During grinding, the components mix intimately and homogeneously to yield structures that can be characterized by X-ray powder diffraction, he explains.
Jones's group uses grinding to screen for cocrystals and to check for polymorph and hydrate stability. For example, simply by changing the liquid used, they have reproducibly made different polymorphs (Chem. Commun. 2004, 890). In a screen for pharmaceutical salts, grinding using methanol had the same efficiency as solution crystallization and even yielded a few new salts (Chem. Commun. 2006, 51).
Because many APIs are optically active, his group "started playing with chiral molecules," Jones says, and tested combinations of racemic, right- and left-handed drugs, and cocrystal formers (Faraday Discuss., DOI: 10.1039/b616399h). One observation with implications for drug forms was that the symmetry of the cocrystal formers changed the thermal properties of the resulting cocrystals.
To test for hydrate stability, Jones and coworkers studied caffeine and theophylline as model APIs and citric acid as the guest (Mol. Pharmaceutics 2007, 4, 347). Liquid-assisted grinding using water proved most useful in making cocrystal hydrates. If they switched to hydrated APIs, however, theophylline formed a stable hydrated cocrystal with citric acid, whereas caffeine and citric acid resulted in an anhydrous one.
Cocrystal studies often generate different solid forms and even new polymorphs. In fact, this is how Zaworortko's group and collaborators at TransForm found the proposed second polymorph of aspirin (J. Am. Chem. Soc. 2005, 127, 16802). "There are always surprises," Bernstein says, based on his experience with oxalic acid (Mol. Pharmaceutics 2007, 4, 355). "This is going to happen because you are creating crystallization conditions that haven't really been tried before."
Given that different methods can produce the same or different cocrystals, most researchers resort to using several means in their discovery efforts. They frequently use grinding, evaporation, sublimation, and melt crystallization. Some focus on solution-based approaches because these are typically used on an industrial scale for crystallization and purification.
"In general, crystallizations by cooling are by far the simplest to develop and scale up, because the fundamentals of the process are thermodynamic and thus scale-independent," AstraZeneca's Black says. And once you know which solid form you want to make, cooling crystallization is the easiest way to ensure that you make these desired crystals, he adds. A common practice is to seed, or initiate, crystallization with small amounts of the desired crystal, a process that also works for cocrystals.
But cocrystallization may run counter to how most people think about crystallization, where the goal is usually to get a pure compound, not two or more coming out of solution together. Black and other researchers emphasize the importance of knowing the system's phase diagram, including the solubility curve, to pinpoint where one needs to be in terms of temperature and composition to make the desired product.
"If you want to control the process to make sure you get the right polymorph or crystallize a salt or cocrystal, you have to know something about how a molecule gets from the solution to the crystal," says Roger J. Davey, professor in the chemical engineering department at the University of Manchester, in England. His research group has been looking at nucleation processes for solid forms, including salts, polymorphs, and cocrystals (Faraday Discuss., DOI: 10.1039/b616164m).
"We try to choose systems where we can do in situ measurements to explore simple questions about the relationship between the solution chemistry in a particular solvent and the crystal structure that appears," he explains. Although one can monitor what's happening, predicting the kinetics and outcome is just too hard.
Lacking the ability to predict outcomes, most pharmaceutical cocrystals are found by taking an API and a range of solvents and potential salt or cocrystal formers and screening combinations. This can be a hit-or-miss approach, but if something does form, it's likely to be fairly robust, or it wouldn't have appeared, many researchers say. At the same time, they admit, depending on the extent of screening, it's possible that something will be missed.
Naír Rodríguez-Hornedo, pharmaceutical sciences professor at the University of Michigan, aspires to a mechanistic, nonempirical approach to understanding cocrystal formation and stability. Thinking practically about how cocrystals could be made and handled industrially, "we want to find methods that are transferable to large scale and understand how vulnerable cocrystals are to processing and storage," she says.

COCRYSTALLIZATION generally involves the slow evaporation of solutions with equimolar or stoichiometric concentrations of the components. In this process, there's always the risk of crystallizing only the single components.
Advertisement
In analogy to precipitating salts by changing the pH, Rodr??guez-Hornedo finds conditions under which cocrystals become less soluble and form from solutions or other wet phases. She has shown mathematically how solubility depends on the equilibrium between the cocrystal and its components (Cryst. Growth. Des. 2006, 6, 592).
Based on this solubility behavior, her group has been able to rapidly generate carbamazepine-nicotinamide and other cocrystals by reaction crystallization (Mol. Pharmaceutics 2006, 3, 362). The process entails either mixing solutions of the reactants to achieve nonstoichiometric amounts or dissolving a nonstoichiometric excess of one in a solution of the other.
At a certain point, the mixture is supersaturated with respect to the cocrystal complex, which precipitates out because it is less soluble than the reactants. Nonstoichiometric concentrations and supersaturation can also be achieved in small quantities of solvent by using solid reactants with different dissolution rates.
"We have not been able to find an existing cocrystal that we cannot make," Rodríguez-Hornedo claims. Her group has shown that even in solvents where the cocrystal is more soluble than the pure API, the reaction can be reversed to form the cocrystal by increasing the concentration of the cocrystal former above a critical value. "The ability to change the thermodynamic relationship between the cocrystal phase and pure API crystal is valuable to control cocrystal formation and stability," she says.
These methods work in both organic and aqueous solvents, although both components must be at least somewhat soluble. Her students typically test methanol, ethanol, and water. The methods are also easily scaled up, applicable to high-throughput crystallization screens, and offer prospects for greener synthetic routes, she points out.
To test stability, Rodr??guez-Hornedo has found that moisture, simply from the air, facilitates cocrystal formation in physical mixtures that contain a deliquescent component, which dissolves as it absorbs moisture in the air. Cocrystal ingredients dissolve in this deliquesced solution and then cocrystallize (Mol. Pharmaceutics 2007, 4, 360). She says moisture can also increase molecular mobility via amorphous phases made during grinding (Pharm. Res. 2006, 23, 2381) or, as she learned in work with collaborators at Schering-Plough and Amgen, in melts and films (J. Pharm. Sci. 2007, 96, 1147).
Meanwhile, other researchers are finding that cocrystal forms can be more stable than the pure API, but the relative stability varies from case to case. Jones and Trask, for example, found that the physical stability of caffeine and the asthma drug theophylline could be increased in cocrystals with oxalic acid (Int. J. Pharm. 2006, 320, 11).
Such factors are important considerations, Rodríguez-Hornedo explains, because drug formulation frequently involves mechanical stress from grinding, milling, or blending. And products may be exposed to different levels of humidity during storage. These changes could either induce cocrystal formation or lead to degradation.
The driver behind making cocrystals is, of course, to improve upon the properties of an API, and many more comparisons are being reported. Rodríguez-Hornedo's group, for example, has found that cocrystals with an ionizable guest can impart pH solubility dependence to nonionizable APIs. "It offers the possibility of tailoring the pH dependence of the dissolution," she notes.
For many years, crystal engineering focused on optical and mechanical properties of materials, says Matthew L. Peterson, a senior scientist and group leader at TransForm. "It has taken some time to recognize that something as simple as dissolution is a very important property to enhance through cocrystals," he says. This realization has opened up a whole new area of looking at what other changes can be made, he adds.
Another driver, suggests TransForm principal investigator Julius F. Remenar, has been the advent of high-throughput drug discovery methods. These methods have resulted in many more poorly soluble compounds that need solid-state engineering. By about 2002, this problem-along with legal, regulatory, and business issues, as well as with emerging work in cocrystals-made the drug industry "ready for cocrystals," he says.

Remenar, Peterson, and coworkers published an early example involving the antifungal drug itraconazole, which is extremely insoluble and not suited to salt formation (a common and generally straightforward route to increase solubility). J&J had made an amorphous form to achieve a usable bioavailability. With a high-throughput crystallization screen, the researchers discovered stable cocrystals using 1,4-dicarboyxlic acids as the cocrystal formers (J. Am. Chem. Soc. 2003, 125, 8456).
With Zaworotko, these scientists have recently compared a carbamazepine cocrystal with the marketed carbamazepine polymorph (Eur. J. Pharm. Biopharm., DOI: 10.1016/j.ejpb.2006.12.016). The drug is an antiepileptic that is water-insoluble and thus requires high doses. It is a favorite in the crystal engineering field because it crystallizes in at least four polymorphs and forms several salts, solvates, and hydrates, though it is a simple molecule with one primary amide hydrogen-bonding site.
Their carbamazepine-saccharin cocrystal could be made by a cooling crystallization from alcohol solution without seeding and was scaled up to a 30-g batch size. With polymorph screening, they found only one polymorph of the cocrystal, which had comparable stability to the marketed form, as well as favorable dissolution properties and comparable oral adsorption.
The TransForm researchers have also looked at cocrystals in aqueous media and in the presence of materials, called excipients, used in formulating products. The API was celecoxib, the well-known analgesic drug that has been studied extensively to improve its absorption. Excipients were found to stabilize a celecoxib-nicotinamide cocrystal with good properties and help direct its conversion into more bioavailable intermediates (Mol. Pharmaceutics 2007, 4, 386).
Formulated cocrystals haven't received much attention, the TransForm scientists say, but are expected to as cocrystals move through drug company pipelines. And although most published examples involve nonproprietary or model APIs, researchers tell C&EN there are indications that several cocrystal drugs are likely to be coming to the market.
Merck researchers recently reported what they say is the first case of a stable cocrystal formed between an API and an inorganic acid (Chem. Commun. 2007, 419). By using a monophosphate salt of the API and phosphoric acid, they say their cocrystal allowed them to avoid the need to use an unstable amorphous drug or salts to get an optimal solid dosage form.
Previously, another Merck group prepared and characterized cocrystals of a phosphodiesterase-IV inhibitor with L-tartaric acid (Cryst. Growth. Des. 2006, 6, 690). The free compound had insufficient bioavailability to develop it for safety studies, but a series of stable nonstoichiometric acid-base cocrystals demonstrated as much as 20-fold improvement in blood plasma concentrations.
Similarly, Pfizer scientists formed complexes of a sparingly soluble anticancer drug candidate containing multiple basic nitrogens and hydrogen-bonding sites with three different dicarboxylic acids (J. Am. Chem. Soc. 2006, 128, 8199). Resulting acid-base complexes, depending on the difference in pKa's of the acids and bases, ranged from neutral complexes to fully ionic salts. Characterization and analysis of these complexes showed significantly enhanced properties needed for drug delivery.
Researchers at Purdue Pharma and SSCI recently reported on the development of a cocrystal to improve the bioavailability of another low-solubility API (Pharm. Res. 2006, 23, 1888). Using a crystal engineering approach, they screened 26 pharmaceutically acceptable carboxylic acids and found a stable API-glutaric acid cocrystal that had an 18-times-greater dissolution rate in water and three-times-higher blood plasma concentrations. Similarly, Scott L. Childs at SSCI and Kenneth I. Hardcastle at Emory University conducted a broad study in which 50 cocrystals of the API piroxicam were made with 23 carboxylic acids (Cryst. Growth Des., DOI: 10.1021/cg060742p).
"COCRYSTAL SCREENING is going to be an empirical process," says G. Patrick Stahly, former chief operating officer of SSCI, "but we start with an initial evaluation of the API in terms of its structure and properties, along with careful selection of potential guests, experimental techniques, and screening protocols." Stahly, who is starting his own consulting firm called Chemfocus, describes the entire solid-form discovery and characterization process in a recent article (Cryst. Growth Des. 2007, 7, 1007).
SSCI is also known for its work on carboxylic acid cocrystals of fluoxetine hydrochloride, the API in the antidepressant Prozac (J. Am. Chem. Soc. 2004, 126, 13335). An estimated 50% of all API salts are made with the chloride ion, and cocrystallization of these salts provides the opportunity to alter physical properties while retaining the salt form in the cocrystal structure, Stahly explains.
"There's also a bit of a debate about whether something is a cocrystal or a salt," he says. In a salt, the components are ionizable and differ enough in pKa that a proton is transferred. When this doesn't happen, as with very weak acids or bases, it's a cocrystal. One can miss forming cocrystals, Stahly warns, if the components are chosen based on large differences in pKa.
Advertisement
But there's another hitch. "If the components are close in pKa, whether the proton is transferred or not depends on the crystal environment," he explains. SSCI researchers explored this "salt-cocrystal continuum" and the influence crystal structure has on the ionization state in 20 complexes of theophylline (Mol. Pharmaceutics 2007, 4, 323).
Similarly, Aakeröy has analyzed 86 salts and cocrystals synthesized from carboxylic acids and N-heterocycles (Mol. Pharmaceutics 2007, 4, 317). "We found that the stoichiometric composition of cocrystals was more predictable than that of certain salts," he says. "In principle, this indicates that cocrystal structures would be more controllable, and, of course, that's key to controlling physical properties."
BUT NOT ALL cocrystals show improved properties, and they are not a panacea for all problems. Although pharmaceutical industry interest is on the rise, many researchers believe drug developers are still waiting for the first big cocrystal product to emerge. It's not yet clear how regulators will treat these new forms and what testing will be required, nor have generic drug firms used a cocrystal form to challenge a related product.
Researchers at TransForm say they have become concerned in the past few years about hype surrounding cocrystals. "When you put it in perspective, the opportunities to make a cocrystal into something useful are rather limited, given the number of possibilities of other forms and how hard it is to actually make a new drug anyway," Peterson says.
The question also arises of the necessity for having a cocrystal form in the first place. "If making a cocrystal of a specific molecule helps to enable an application or solve a problem, then it's the right thing to do," Remenar adds. "But you don't design a molecule in the real world simply because you are looking for a cocrystal strategy."


Join the conversation
Contact the reporter
Submit a Letter to the Editor for publication
Engage with us on Twitter