Advertisement
Grab your lab coat. Let's get started
Welcome!
Welcome!
Create an account below to get 6 C&EN articles per month, receive newsletters and more - all free.
It seems this is your first time logging in online. Please enter the following information to continue.
As an ACS member you automatically get access to this site. All we need is few more details to create your reading experience.
Not you? Sign in with a different account.
Not you? Sign in with a different account.
ERROR 1
ERROR 1
ERROR 2
ERROR 2
ERROR 2
ERROR 2
ERROR 2
Password and Confirm password must match.
If you have an ACS member number, please enter it here so we can link this account to your membership. (optional)
ERROR 2
ACS values your privacy. By submitting your information, you are gaining access to C&EN and subscribing to our weekly newsletter. We use the information you provide to make your reading experience better, and we will never sell your data to third party members.
Environment
Green For The Greater Good
Presidential awards honor chemical advances that promote pollution prevention and sustainability
by Stephen K. Ritter
July 9, 2007
| A version of this story appeared in
Volume 85, Issue 28
WHEN IT COMES to green chemistry, big numbers are a big deal. Consider 145 million lb of hazardous chemicals and solvents eliminated from industrial use each year. That's enough material to fill almost 700 railroad tank cars, which would make up a train nearly 9 miles long. Or how about 55 million gal of water saved each year, which adds up to the amount of water used by 2,100 people. Then there's 57 million lb of carbon dioxide no longer released to the atmosphere each year, the equivalent of taking 6,000 cars off the road. These numbers represent the savings of the 57 groundbreaking technologies that have received Presidential Green Chemistry Challenge Awards since the program's inception in 1995.
Richard Engler, director of the Environmental Protection Agency's Green Chemistry Program, which administers the awards, takes some pride in keeping track of those statistics. "Through 2006, Challenge winners have made nearly 750 million lb of progress," Engler says. It's the result of "cleaner, cheaper, and smarter chemistry."
EPA's program supports companies in developing chemical products and processes that require fewer reagents, less solvent, and less energy while generating less waste. Even as chemists and chemical engineers are designing these processes and products to have low toxicity and be safer to use than their predecessors, they also are shooting for profitability.
This year's class of five award winners, drawn from more than 100 nominees, now is set to start adding to the big-number totals. They were recognized for their scientific advances during a ceremony held on June 26 at the National Academy of Sciences in Washington, D.C.
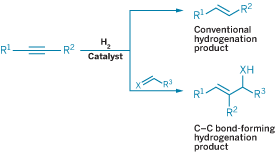
Making bonds is the primary job function of many chemists. So when a chemist devises a new way to make bonds, it becomes a reason to celebrate. Take Michael J. Krische of the University of Texas, Austin. His work was celebrated with the Academic Award for developing a broad new class of hydrogenation reactions that form carbon-carbon bonds.
Conventional hydrogenation reactions typically result in formation of carbon-hydrogen bonds. But in Krische's reactions, two or more unsaturated organic molecules, when exposed to hydrogen gas in the presence of a metal catalyst, are hydrogenated and join together via a new C-C bond to create a single, more complex product. The reactions perfectly satisfy the green chemistry principle of atom economy because all atoms present in the starting molecules, including the hydrogen, appear in the final product and the reactions don't generate any by-products or waste, he says.
"Elemental hydrogen is the cleanest, most cost-effective reductant available," Krische notes. "Accordingly, hydrogenation reactions are practiced industrially on a vast scale."
But prior to his work, hydrogen-mediated C-C bond formations were limited to reactions requiring carbon monoxide as a coupling partner, Krische says. One example is the alkene hydroformylation reaction, typically used to make aldehydes, in which a formyl group (CHO) and a hydrogen atom are added across a double bond. Another example is the Fischer-Tropsch reaction, in which carbon monoxide and hydrogen are transformed into various hydrocarbons.
Both of those industrial processes were developed more than 70 years ago, Krische notes. But until he came along, no one had carried out a systematic study to determine if reactive intermediates in catalytic hydrogenations could be intercepted and rerouted to selectively form C-C bonds.
"Given the impact of these processes, systematic efforts toward hydrogen-mediated C-C bond formations beyond carbon monoxide coupling are warranted and long overdue," he says.
Krische's hydrogen-mediated couplings take advantage of rhodium or iridium catalysts and avoid preformed organometallic reagents, such as Grignard (RMgBr), Gilman [(CH3)2CuLi], lithium, and zinc reagents. These organometallic reagents typically are highly reactive and moisture-sensitive—in some cases catching fire when exposed to air. An added advantage for Krische's work comes into play when using chiral catalysts, which permit enantioselective C-C bond formation. The increased safety, cost-effectiveness, and by-product-free nature of the new reactions "auger well for their use in the chemical industry," he says.
Krische's group is applying the strategy to make myriad types of compounds. In one recent example, Krische and coworkers coupled acetylene to N-arylsulfonyl imines (aldimines) via an enantioselective rhodium catalyst to form chiral allylic amines (J. Am. Chem. Soc. 2007, 129, 7242). In another example, in which they used an iridium catalyst for the first time with their strategy, the team coupled alkynes to α-ketoesters to form α-hydroxyesters (J. Am. Chem. Soc. 2007, 129, 280).
"Hydrogenation has been utilized almost exclusively as a method of reducing organic molecules," Krische says. "My group's efforts reveal that hydrogenation can be utilized as a method of C-C bond formation across a broad range of reactions."
SINCE THE 1940s, the wood composites industry has used phenol-formaldehyde and urea-formaldehyde resins as adhesives to bind wood pieces and fragments into composites, such as plywood and particleboard. Formaldehyde now is categorized by environmental protection agencies as a probable human carcinogen. Thus, the wood products and other industries are under pressure from consumer and environmental groups—and themselves—to reduce its use. In April, for example, California passed a state regulation to reduce formaldehyde emissions from wood composites.
To address these concerns, Kaichang Li of Oregon State University, Columbia Forest Products, and Hercules, winners of the Greener Synthetic Pathways Award, joined forces to commercialize an adhesive made from soy flour as an environmentally friendly replacement for conventional formaldehyde-based adhesives. In 2006, Columbia used the new adhesive, sold under the PureBond brand, to replace more than 47 million lb of formaldehyde adhesives in the 9 billion-lb North American market, Li says.
The path to the soy-based adhesives started several years ago with a trip to the beach, where Li was hoping to catch a few crabs. As a wood chemist specializing in adhesives, Li took notice of the ability of mussels to adhere to rocks in the tide pools. "I was amazed," he says. "Typically, an adhesive needs a clean, dry surface. But mussels seemingly have the ability to stick to anything, anywhere."
Li and his colleagues began to wonder how the mussel's adhesion could be so strong. After studying mussels, fungi, and natural products from such organisms, Li and his group began to find some answers in catechol, amine, and other functional groups common to the proteins in natural adhesives. Then, while eating tofu one day, it dawned on Li that soy protein was abundantly available, and its amino acids likely could be modified to mimic that of the mussel adhesion protein. The Oregon State researchers knew that the mussel protein contains large amounts of 3,4-dihydroxyphenylalanine, lysine, and cysteine, while soy protein does not. On the other hand, soy protein contains carboxylic acid groups owing to its aspartic acid and glutamic acid units. The chemists started modifying soy protein with catechol, amino, and mercapto groups while blocking the carboxylic acid groups with curing agents. In this way, they created the adhesive they were looking for: a very strong, water-insoluble compound with a three-dimensional molecular network.
Li turned to Columbia to begin testing the new adhesive. Hercules joined the team to provide a polyamidoamine-epichlorohydrin curing agent and the expertise to apply it to commercial production of plywood. The curing agent already is used in the paper industry for adding strength to paper towels, facial tissue, and milk cartons.
"Our company had been moving to reduce formaldehyde emissions for years," according to Steve Pung, Columbia's vice president of technology. "When we learned about Li's innovation using soy flour, we instantly recognized its potential to eliminate formaldehyde from our production process. It was very easy for us to commit to funding the research and development."
It was also an easy decision for Hercules "to make the commitment to invest resources for advancing this discovery to a commercially viable technology," says Charles L. Robinson, general manager of Hercules' Ventures Group. "Promoting the use of environmentally friendly and sustainable chemical products and processes was and is the right decision for Hercules and the wood paneling industry."
Columbia is North America's largest manufacturer of hardwood plywood and veneer. All of the company's plywood plants now use the new adhesive, which reduces emissions of volatile organic compounds from each site by 50-90%, Li emphasizes. Columbia has also replaced formaldehyde-based adhesives in its particleboard plant, he notes. The company additionally is seeking arrangements with other manufacturers in the hope of expanding the industry's adoption of the adhesives.
"With this technology, those who make and use furniture, kitchen cabinetry, and other wood-composite materials have a high-performing formaldehyde-free alternative," Li says. "Replacing the toxic adhesives greatly enhances the global competitiveness of the U.S. wood composites industry."

HYDROGEN PEROXIDE is an important industrial bleaching and oxidizing reagent that is an environmentally friendly alternative to chlorine and chlorine-containing chemicals. The catch is that H2O2's indirect manufacturing process is complex and energy-intensive, and it generates undesirable by-products, making H2O2 more expensive to produce.
That's why chemical companies have been eager for several decades to develop a simpler, large-scale synthesis of H2O2 directly from hydrogen and oxygen that produces only water as a by-product. Headwaters Technology Innovation (HTI), Lawrenceville, N.J., has done just that and was recognized with the Greener Reaction Conditions Award for its process that utilizes an advanced metal nanocatalyst technology. By cutting capital costs as much as 50%, the technology is expected to generate a greener and competitively priced supply of H2O2, notes Bing Zhou, president of HTI.
The traditional commercial process for making H2O2 requires a continual cycle of catalytic reduction and oxidation of anthraquinone in an organic solvent using H2 and O2, Zhou notes. The peroxide is separated from solution and concentrated by vacuum distillation in an energy-intensive process. Although the reaction solution is recycled, the process generates a waste stream of undesirable quinone by-products that require treatment and disposal, he says.
HTI developed a palladium-platinum catalyst, part of its NxCat Next-Generation Nanocatalyst Technology, which eliminates all the hazardous reaction conditions, chemicals, and by-products of the existing H2O2 process, Zhou explains. HTI's nanotechnology approach, 17 years in the making, "is the key for enabling our cutting-edge process to compete with the commercial anthraquinone process," he says.
Zhou and his colleagues engineered a set of polymer templates containing multiple functional groups that have different affinities for palladium and platinum, he explains. As catalyst particles form, the templates precisely control the catalyst's palladium-to-platinum ratio, surface structure, and particle size.
The company's approach further allows uniform dispersion of the Pd-Pt nanoparticles on solid supports, avoiding aggregation of the nanoparticles and permitting a low loading of the catalyst, he says. Tight bonding of the nanoparticles on the support surface also helps prevent loss of the expensive metals and maintains catalyst stability for up to three years of use.
The uniform 4-nm particle size safely enables a high peroxide production rate with an H2 concentration below 4%, which avoids a flammability hazard, Zhou says. Larger particles have lower catalytic activity, he adds, while smaller 2-nm particles, because of their surface properties, end up producing water instead of H2O2. The Pd/Pt ratio and the optimal arrangement of metal atoms on the particle surfaces leads to 100% selectivity for the H2O2 product, compared with about 80% selectivity for other direct processes.
"NxCat enables a simpler manufacturing process to produce a competitively priced supply of H2O2 that could speed its inclusion in industrial processes that now use more environmentally deleterious chemicals," Zhou says.
In 2004, Headwaters partnered with Degussa, a world leader in H2O2 production, to build a demonstration plant using the nanocatalyst. The plant started running in October 2006, and it's allowing the joint-venture partners to collect the data necessary to design a full-scale plant, Zhou notes. The companies plan to begin construction of a commercial H2O2 plant in 2009, with an eye toward producing propylene oxide from propylene for the polyurethane market.
Advertisement
NO ONE WANTS to think about the need to have a skin graft, reconstructive surgery requiring a bone or ligament transplant, or a health condition that calls for an implanted medical device. But should one of these treatments be necessary, you would like to take comfort in knowing that the material going into your body is sterilized and safe to use.
The most common medical sterilization techniques—exposure to ethylene oxide gas or irradiation with gamma rays—work well on a large scale for instruments, equipment, clothing, and bandages. But they are not suitable for more delicate biological samples such as bone and tissue. These materials instead are disinfected by rinsing with peroxides, which can leave behind some residues.
Enter NovaSterilis, Ithaca, N.Y., which was presented with the Small-Business Award for developing an inherently safer and gentler sterilization method that employs supercritical carbon dioxide. The technology is a "terminal" sterilization process, which means the treatment occurs after the material is in its final packaging, notes Bruce Ganem, a company cofounder and a chemistry professor at Cornell University.
The company's commercially available instrument, the Nova 2200, currently is being marketed to tissue banks for sterilizing packaged bone and tissue transplants prior to shipping to hospitals, Ganem says. NovaSterilis plans to expand the applications to include sterilizing drug-coated cardiovascular stents, arthroscopic surgical instruments, and whole-cell vaccines.
NovaSterilis got started by licensing a patent for sterilizing biodegradable polymers, an invention of chemical and biological engineer Robert S. Langer and colleagues at Massachusetts Institute of Technology. When challenged with finding a suitable method to sterilize the polymers, Langer's group turned to supercritical CO2, a dense liquid-gas phase of CO2 that has useful solvent properties. Supercritical CO2 can be used to extract caffeine from coffee, for example. NovaSterilis scientists spent the past few years expanding and optimizing Langer's technique for biomedical applications.
The supercritical CO2 sterilization process, carried out at 30-45 °C and moderate pressure (1,200 psi), requires the presence of a peroxide additive and a small amount of water, Ganem explains. Before treatment, samples are placed in coated plastic bags made of DuPont's Tyvek high-density polyethylene fiber.
"Tyvek is ideal for allowing gases in but keeping viruses and bacteria out," Ganem says. "In as little as one hour, the Nova 2200 totally inactivates microbes, including bacterial spores."
The mechanism of bacterial inactivation is not well-understood, Ganem points out, but it doesn't appear to involve rupturing bacterial cells (lysis) or wholesale degradation of bacterial proteins. He speculates that CO2 and water form a small amount of carbonic acid (H2CO3), a reaction that may be amplified by the presence of the peroxide. The supercritical CO2 permeates the bacterial cells, carrying the carbonic acid along with it. The decrease in pH may be what spells doom for the bacteria, he says.
Supercritical CO2 offers several advantages over the current sterilization approaches, Ganem notes. Tissue banks typically sterilize samples by "aseptic washing," a process in which the bone or tissue is soaked or rinsed with a peroxide, such as peracetic acid (CH3CO3H). Samples may additionally be treated by exposure to gamma rays from cobalt-60. In the case of sterilized tendon, NovaSterilis' process removes unwanted residues—presumably impurities—which results in greater aesthetic appeal of tissue grafts compared with aseptic washing and reduces the amount of required chemicals, he says.
Of the other methods, ethylene oxide is "terribly toxic," he notes, in addition to being flammable and a readily polymerized gas. It requires an outgassing procedure to remove residual gas from the packaging, and polymer residues remain in the sterilized material, increasing the risk of side effects. It's now banned in the European Union, he adds. Gamma radiation is highly penetrating and indiscriminately lethal to all cells, he says, thus causing radiation damage that weakens materials and raises safety issues for technicians.
NovaSterilis is just starting to sell Nova 2200 units to tissue banks, with a price tag of $140,000. "It's a lucrative market segment," Ganem says. According to the American Association of Tissue Banks, some 1.5 million bone and tissue grafts are distributed by 1,100 tissue banks in the U.S. each year. NovaSterilis estimates that tissue banks spend about $400 million annually on sterilization procedures.
The Food & Drug Administration doesn't fully regulate tissue banks, because they are nonprofit organizations, Ganem adds. So sterilization of bone and tendon samples was the quickest route to market for NovaSterilis. But the company will need to get FDA approval to use the technology for sterilizing drug-delivery devices, medical instruments, and vaccines, a process that could take up to three years, he says.

EVERYONE LIKES to sit on a comfy couch to read or watch a little TV, to have a restful night's sleep in bed, or to take an enjoyable ride in their car. One key to those activities is the quality of the foam cushioning behind the scenes. That's where Cargill, selected for the Designing Greener Chemicals Award, fits in.
Cargill scientists, working in conjunction with chemists at Pittsburg State University's Kansas Polymer Research Center, have come up with a way to prepare polyols from soybean oil rather than from petroleum. Polyols are monomers containing multiple alcohol groups that typically are copolymerized with an isocyanate monomer to make flexible and rigid polyurethane foams used in furniture, bedding, and automobiles.
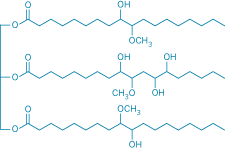
Cargill's approach is not the first commercial example of making a product from a renewable agricultural resource, but it's one of the first to do so in a major market segment. Even the idea of replacing petroleum-based polyols with biobased polyols is not new, but the poor performance, discoloration over time, and objectionable odor—a smell like burned popcorn—of previous efforts restricted them to limited markets, according to Yusuf Wazirzada, Cargill's business development manager for its BiOH brand of polyols. The BiOH name derives from bio, as in biobased, and the OH chemical symbol for an alcohol group.
Petrochemical-based polyols are made from ethylene oxide or propylene oxide precursors, Wazirzada explains. Cargill makes BiOH polyols by converting the carbon-carbon double bonds in the unsaturated fatty acid chains of triglycerides in soybean or other vegetable oils to alcohol and methoxy groups. BiOH polyols, in turn, are used like petroleum-based polyols to make polyurethanes, Wazirzada says.
Cargill estimates that each 1 million lb of BiOH polyols incorporated into polyurethane saves nearly 700,000 lb (2,200 barrels) of crude oil, reducing dependence on petroleum. And replacing petroleum-based polyols with BiOH polyols reduces total energy use by 23% and CO2 emissions by 36%. BiOH polyols also have low flammability, low volatility, and are relatively nontoxic, providing safer handling relative to petroleum-based polyols, Wazirzada adds.
Besides reducing the "environmental footprint" of polyurethanes, BiOH polyol foams "set a new standard for consistent quality with low odor and color," Wazirzada says. "Foams containing BiOH polyols retain their white color longer without ultraviolet stabilizers. They also have comparable performance to foams containing only petroleum-based polyols in standard tests."
In large flexible foams, such as those used in furniture and bedding, BiOH 5000 polyols provide processing versatility, improved comfort, and reduced variations in density, he says. In molded foams, such as automotive seating and headrests, BiOH 2100 polyols enhance load-bearing or hardness properties relative to conventional polyol foams.
Polyurethanes make up a $20 billion global industry, and the U.S. market for polyols to produce polyurethanes is more than 3 billion lb and growing steadily, according to Cargill. Several major manufacturers already use the company's polyols to replace petroleum-based feedstocks. The list includes Woodbridge Group, a leading provider of molded automotive polyurethane products; Hickory Springs Manufacturing, a foam producer for upholstered furniture and bedding; and Flexible Foam Products, a supplier of polyurethanes for furniture and automotive applications.
Cargill aims to eventually achieve a 100% market replacement for rigid and flexible polyurethane foams. The company also plans to expand use of the BiOH polyols to polyurethane coatings, adhesives, sealants, and elastomers, Wazirzada notes.
Cargill's BiOH polyols, which went from concept to commercial sales in just 26 months, "represent not just a technical success and not just an environmental success, but a commercial success," says Ronald L. Christenson, Cargill's chief technology officer. "In solving tough technical challenges and quickly commercializing the product, the Cargill team is making the business case for green chemistry."
Only by creating products that work as well or better than products currently made from petroleum—and doing so in a cost-competitive manner—will "green products ever gain a significant toehold," Christenson adds. But profit isn't Cargill's only motive. Green chemistry fits with the company's citizenship goals for having a positive impact on society and the environment, "a mind-set that must be embedded in all our business practices," Christenson says.





Join the conversation
Contact the reporter
Submit a Letter to the Editor for publication
Engage with us on Twitter