Advertisement
Grab your lab coat. Let's get started
Welcome!
Welcome!
Create an account below to get 6 C&EN articles per month, receive newsletters and more - all free.
It seems this is your first time logging in online. Please enter the following information to continue.
As an ACS member you automatically get access to this site. All we need is few more details to create your reading experience.
Not you? Sign in with a different account.
Not you? Sign in with a different account.
ERROR 1
ERROR 1
ERROR 2
ERROR 2
ERROR 2
ERROR 2
ERROR 2
Password and Confirm password must match.
If you have an ACS member number, please enter it here so we can link this account to your membership. (optional)
ERROR 2
ACS values your privacy. By submitting your information, you are gaining access to C&EN and subscribing to our weekly newsletter. We use the information you provide to make your reading experience better, and we will never sell your data to third party members.
Materials
Materials Science Stars In Boston
Action-packed presentations feature wasps, spiders, and nanotech
by Bethany Halford
January 15, 2007
| A version of this story appeared in
Volume 85, Issue 3

THERE WERE NO PALM TREES, paparazzi, or starlets sporting oversized sunglasses, but the annual fall meeting of the Materials Research Society (MRS), held on Nov. 27-30, 2006, in Boston, had the undeniable air of Hollywood about it. It could have been the unseasonably warm weather or the symposium rooms packed like a blockbuster film premiere, but it's more likely that the extra glamour arrived courtesy of MRS's first-ever film festival.
Coinciding with the recent YouTube craze, the special event proved that the folks at MRS have their finger on the pulse of popular culture. A dozen established and aspiring filmmakers submitted short features, which played for conferees on a continuous loop in the meeting's exhibition hall. The cinema had something for everyone, from straight-laced science documentaries to gecko-style wall scaling and dancing cartoon data bits.
Attendees could vote for their favorite films. And guided by those votes, a committee chose six winning films: three from professional filmmakers and three from amateurs working on a shoestring budget. Winners received cash prizes and had their films shown on the big screen during lunch on the meeting's final day. (Several of the award-winning filmmakers graciously agreed to screen their motion pictures at http://pubs.acs.org/cen/science/85/8503sciweb.html.)
Besides the riveting films, the meeting's 5,400 or so conferees had an opportunity to learn about the latest materials research from more than 5,000 papers that were presented. As the following highlights suggest, there was something to satisfy even the pickiest of critics, with talks on everything from nanoscale electronics to wasp anatomy.
Introducing nanopiezotronics
THE NIGHT BEFORE giving a talk at an MRS meeting, most speakers review their notes, check their presentations for typos, and try to get some beauty sleep. But Zhong Lin Wang had slightly more ambitious plans on the eve of his presentation. He decided to invent a new word: nano-piezotronics.
Wang, director of Georgia Institute of Technology's Center for Nanostructure Characterization, said his new addition to the nano lexicon arose out of an attempt to describe the use of piezoelectric semiconductors to make novel nanoscale electronic devices.
Nanopiezotronics is going to be important for nanoscale electronics, Wang explained, because in today's electronic devices, the size of the power source dictates the size of the system. "There are a lot of neat nanodevices out there, but there are no nanobatteries," he said. "What we're doing is going from a nanodevice to a self-powered nanosystem, which includes nanodevice components as well as a nanoscale power source."
The key to Wang's tiny power generators is zinc oxide's combination of piezoelectric and semiconducting properties. Piezoelectric materials possess the ability to generate a voltage in response to mechanical stress. Bending a ZnO nanowire, for example, transforms the mechanical energy of bending into electrical energy. Bending an array of ZnO nanowires might generate enough electricity to power a nanodevice (Science 2006, 312, 242).
Dim the lights and get your popcorn ready. Award-winning films from the MRS film festival can be seen on C&EN Online.
A ZnO nanowire can also be used as a semiconductor, one of the core components in a field-effect transistor (FET), the key switching structure in nanoscale sensors and devices. In this capacity, the nanowire bridges the source electrode on one end and the drain electrode on the other. Applying an electrical potential to a third electrode, known as the gate, controls whether current can pass through the semiconductor.
Taking advantage of the piezoelectric properties in a ZnO nanowire, Wang's group was able to create an FET without a gate electrode. That's because ZnO does the gate's job too, Wang explained, bending or straightening in response to pressure. This movement creates a potential across the nanowire, controlling the current flow between the source and the drain.
Wang and colleagues, including postdoc Xudong Wang and graduate student Jinhui Song, have already used this architecture to create pressure sensors with the ability to measure forces in the nanonewton range (Nano Lett. 2006, 6, 2768). That's sensitive enough to weigh a dust mite.
"The potential applications are tremendous," Wang enthused. Nanopiezotronic devices could some day cover airplanes and space shuttles in a skin of wireless pressure sensors, he said. Nanopiezotronic generators could be put into knee patches on military fatigues and the soles of combat boots, creating power for soldiers on the march.
Don't plan on plugging your laptop into your running shoes just yet. Wang noted that the technology is still in its early stages. But he thinks nanopiezotronics is going to be more than just a new word. "This should be a whole new field," he remarked.
Tiny tuning-fork sensors
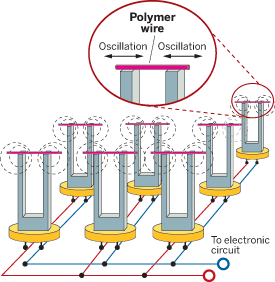
BY MINING THE INNER WORKINGS of an everyday wristwatch, Nongjian Tao, a chemistry professor at Arizona State University, has built a novel and inexpensive sensor that can detect improvised explosives and environmental pollutants and also distinguish between different brands of beer.
The key component in the sensing system is a quartz tuning fork, which is more commonly used to keep time in wristwatches. Because quartz is a piezoelectric material, it can turn mechanical energy into electrical energy and vice versa. In a wristwatch, a quartz tuning fork oscillates at a regular frequency in response to voltage.
"We were trying to develop a sensor that can measure the force of a single molecule," Tao explained. One way to measure such a small force, he reasoned, would be to develop a system that could convert a chemical binding event into an electrical signal. Working with research assistant professor Erica S. Forzani and graduate student Francis Tsow, Tao figured out a way to use the quartz tuning fork's reliable oscillation to detect a chemical binding event.
To do so, they stretched a thin polymer wire across the tuning fork's tines. As the fork oscillates, the wire stretches and compresses. When the wire absorbs analyte molecules, its mechanical properties change. This change, in turn, alters the tension of the wire and, consequently, its oscillation, which is easy to detect electrically.
Tao's group selects their polymeric materials according to the chemical vapor they'd like to detect. Poly(vinylphosphonic acid) works well for monitoring relative humidity, and wax is good at sniffing out nitrobenzene derivatives. The technique is particularly useful for detecting low-molecular-weight molecules that might elude sensors that detect chemicals according to changes in mass or surface stress, Tao noted.
With a polymer wire made from Apiezon wax, Tao's group was able to detect p-nitroethylbenzene (a derivative of the pollutant ethylbenzene frequently found in the atmosphere) at parts-per-billion levels. Using a quartz tuning fork array with different types of polymer wires and pattern recognition techniques, they determined the ethanol concentration in unlabeled bottles of beer, making it possible to distinguish a light beer from a more potent brew (Anal. Chem. 2005, 77, 2700).
Even though it's very sensitive, Tao noted, the system remains reliable in real-world situations. "The tuning fork is remarkably stable under a variety of temperatures, pressures, and humidities," he said. "That's why it's used in watches."
And because the tuning forks cost only 10 cents each, the entire system can be assembled for about $15. "The battery is the most expensive item," Tao quipped.
Untangling spider silk's structural mysteries

THE 6,000 LB OF GOAT MILK sitting in Randolph V. Lewis' laboratory freezer at the University of Wyoming takes up a lot of space. But it's only one part of the biochemistry professor's effort to understand spider silk and replicate its remarkable properties.
Of course, the caprine concoction is no ordinary goat milk. It comes from Montreal-based Nexia Biotechnologies, a company that made headlines a few years ago when it announced it had created genetically modified goats that produce milk laden with spider silk proteins. And understanding the chemistry and biophysics behind spider silk's remarkable properties has been at the heart of Lewis' research for nearly two decades (Chem. Rev. 2006, 106, 3762).
To begin with, spiders don't make just one type of silk, Lewis noted. The ingenious arachnids produce silk in up to six varieties of varying strength and elasticity. Of these, Lewis said, dragline silk has attracted the most commercial interest.
Spun by orb-web-weaving spiders for their web's framework and for the spiders to catch themselves during a fall, dragline silk is about as strong as the bulletproof polymer Kevlar. Unlike Kevlar, however, dragline silk is also extremely elastic, which is why it takes 10 times more energy to break dragline silk than it does to break Kevlar.

Spider silk is made almost entirely of protein, so from a molecular standpoint, what is it that makes dragline silk so strong and also so elastic?
Thanks to fiber diffraction studies, scientists have long known that spider silk proteins possess crystalline regions of β-sheets. "The problem is that almost all the information we get is on those crystalline regions," Lewis explained. Although those β-sheets are important, they don't tell the whole story. The protein structure that gives spider silk its amazing elasticity is still something of a mystery.
Because spider silk is insoluble in all but a few organic solvents, scientists can't use traditional protein characterization methods, such as X-ray crystallography and nuclear magnetic resonance spectroscopy, to suss out its structure. So Lewis' team decided to see what they could learn from examining the silk protein's amino acid sequence.
Lewis pointed out that one of dragline silk's proteins contains stretches of polyalanine of up to seven residues alternating with repeating sequences of three residues: two glycines (GG) and a third amino acid (X) that is either tyrosine, leucine, or glutamine.
On the basis of computer modeling, Lewis thinks this repeating GGX motif forms a helical arrangement in which each triplet makes a complete turn in the helix. In that structure, all the X residues extend outward from one side of the helix. The models also show that if two antiparallel strands of this helix come together, their X residues can interdigitate like a zipper. "We believe this is a key factor in terms of tensile strength," Lewis said.
The modeling studies have also led Lewis' group to propose that dragline silk's elasticity comes from a pentapeptide motif of glycine-proline-glycine (GPG) and two other amino acids (XX). This GPGXX sequence is thought to form a β-turn, and multiple β-turns from GPGXX take the shape of a springy β-spiral. "Looking at the structure," Lewis said, "you can imagine that if you pull, that spiral stretches."
Comparing the sequences of a variety of natural spider silks, Lewis noted that proteins lacking this GPGXX elastic segment can't be stretched beyond 5% of their original length. If a silk protein has nine of the elastic segments, however, its elasticity is 20 to 30%. And if the protein has 60 GPGXX repeats, its elasticity is more than 200%.
To test these computational models experimentally, Lewis' group, including postdoc Florence Teulé and undergraduate Alyssa Cooper, has made 18 different variations on the dragline silk protein. They tweak amino acids to see which factors are the most important in terms of strength and elasticity in spun fibers.
The wondrous construction of a wasp's waist

THE LATEST RESEARCH PROJECT in Christopher Viney's lab started in a most unusual fashion when his physics lecture was interrupted by a loud thwack from the rear of the classroom. "I was hoping it wasn't one of my students whacking his head on the desk in frustration," joked Viney, an engineering professor at the University of California, Merced.
As it turns out, the disruption had arisen out of an act of extermination, rather than frustration, and at the end of the class, the student responsible for the noise approached Viney with a dead mud dauber wasp. "I believe he said something like, 'You work with weird things. Here's a sample,' " Viney recalled.
Having investigated giraffe sweat and slug mucus in the past-projects that Viney told C&EN have a certain recruitment value with students—he couldn't help but be struck by the long and slender shape of the wasp's waist or petiole, as it's known in entomology circles.
Advertisement
In an adult mud dauber wasp, or Sceliphron caementarium, the petiole can grow to lengths exceeding 5 mm, even though its diameter remains a trim 0.5 mm. Although the petiole is basically a hollow tube, it performs a number of functions for the wasp. It serves as a digestive and circulatory connection between the thorax and the abdomen and protects nerve tissue.
Despite its obvious importance to the wasp, the petiole has a deceptively delicate appearance. Viney remembered looking at the recently deceased wasp and thinking, "I would like to know just a little bit more about what's going on in the waist of that wasp."
With the help of Emily Reed, an undergraduate biology major at UC Merced, he decided to take a closer look at the petiole's structure. When they examined a cross-section of the petiole with a scanning electron microscope, they observed a microstructure made of 43 concentric layers of material. Multilayered structures tend to be quite tough, Viney explained, because they deflect cracks rather than letting them propagate.
An even closer look at the petiole's nanostructure with a transmission electron microscope revealed a corrugated structure, similar to that of cardboard, in the medium between the petiole's structural layers. In cardboard, this corrugation makes bending difficult in some directions but not in others, Viney explained. But in the petiole, the corrugation is coiled so that it resists kinking in all directions.
Viney thought it would be interesting to measure the petiole's mechanical properties, but his lab was undergoing construction, and he didn't yet have the appropriate equipment. Still, with a steady hand and some superglue, Viney managed to construct a cantilever out of the petiole, and with a little engineering know-how, he got a reasonably accurate measure of its stiffness.
He found that the petiole's stiffness is similar to that of the aluminum used in airplanes. "That's not all that surprising," he joked, "since these things have to fly." Next, he and Reed hope to build a macroscopic model based on the petiole's coiled, corrugated structure to see how that design influences stiffness.
Read More
- Materials At The Movies
- Dim the lights and get your popcorn ready. Winners of the Materials Research Society's first ever film festival have graciously agreed to share their award-winning motion pictures with C&EN Online.




Join the conversation
Contact the reporter
Submit a Letter to the Editor for publication
Engage with us on Twitter