Advertisement
Grab your lab coat. Let's get started
Welcome!
Welcome!
Create an account below to get 6 C&EN articles per month, receive newsletters and more - all free.
It seems this is your first time logging in online. Please enter the following information to continue.
As an ACS member you automatically get access to this site. All we need is few more details to create your reading experience.
Not you? Sign in with a different account.
Not you? Sign in with a different account.
ERROR 1
ERROR 1
ERROR 2
ERROR 2
ERROR 2
ERROR 2
ERROR 2
Password and Confirm password must match.
If you have an ACS member number, please enter it here so we can link this account to your membership. (optional)
ERROR 2
ACS values your privacy. By submitting your information, you are gaining access to C&EN and subscribing to our weekly newsletter. We use the information you provide to make your reading experience better, and we will never sell your data to third party members.
Materials
H-Bonds Control Amyloids
December 24, 2007
| A version of this story appeared in
Volume 85, Issue 52
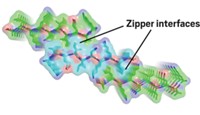
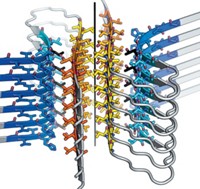
Amyloid protein fibrils are best known as bad actors in Alzheimer's and related diseases. But they can also serve functional roles, as bacterial coatings, for example, suggesting that amyloids might become useful as designed synthetic materials. Although the flexibility or rigidity of the fibrils varies over a range of four orders of magnitude, the factors determining such properties are not well-known. A major factor controlling this flexibility or rigidity turns out to be the fibrils' hydrogen-bonding networks, according to Christopher M. Dobson, Mark E. Welland, and coworkers at the University of Cambridge (Science 2007, 318, 1900). The researchers found that a key determinant of amyloid structure is the strength of H-bonds that form among stacked β-sheets in the common core structure of the highly ordered fibrils (shown, H-bonds are yellow). Variable side-chain interactions also have an important influence, but to a lesser degree. These conclusions could lead not only to a better understanding of the role of amyloid fibrils in disease but also to the greater use of such structures "both in biotechnology and as nanoscale materials," the researchers note.




Join the conversation
Contact the reporter
Submit a Letter to the Editor for publication
Engage with us on Twitter