Advertisement
Grab your lab coat. Let's get started
Welcome!
Welcome!
Create an account below to get 6 C&EN articles per month, receive newsletters and more - all free.
It seems this is your first time logging in online. Please enter the following information to continue.
As an ACS member you automatically get access to this site. All we need is few more details to create your reading experience.
Not you? Sign in with a different account.
Not you? Sign in with a different account.
ERROR 1
ERROR 1
ERROR 2
ERROR 2
ERROR 2
ERROR 2
ERROR 2
Password and Confirm password must match.
If you have an ACS member number, please enter it here so we can link this account to your membership. (optional)
ERROR 2
ACS values your privacy. By submitting your information, you are gaining access to C&EN and subscribing to our weekly newsletter. We use the information you provide to make your reading experience better, and we will never sell your data to third party members.
Materials
A Materials Feast In Boston
Scientists dig in to sessions on unconventional electronics, nanoparticles, and polymers
by Bethany Halford
December 24, 2007
| A version of this story appeared in
Volume 85, Issue 52
WHILE MOST OF AMERICA thinks of Thanksgiving as a traditional time to indulge, materials scientists know, intellectually speaking, that the real feast arrives the week after the November holiday. That's when the Materials Research Society (MRS) serves up its annual fall meeting, offering materials researchers from around the world a vast repast of science to sample.
More than 5,100 conferees attended this year's meeting, which featured approximately 4,600 oral and poster presentations in a smorgasbord of 43 technical sessions. Attendees also got a special treat with the meeting's "Science as Art" competition, where entrants could show off the artistic side of their research. Winning images from the competition, including nanoscale explosions, otherworldly landscapes, and the world's tiniest fuzzy dice can be seen on C&EN Online at www.cen-online.org/science/85/8552sci2web.html
As the following highlights suggest, the meeting, held Nov. 26–30 in Boston, provided plenty of food for thought with topics ranging from organic electronics to polymers to nanotechnology.
Nanoparticles In Disguise
BY DRESSING a nanoparticle in a virus's capsid coat, chemists at Indiana University, Bloomington, have discovered a way to spy on these infectious agents, learning how they assemble and how they travel throughout an organism.
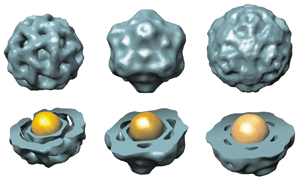
A virus's capsid is more than just a protective covering for its nucleic acid core: This protein sheath also controls the virus's ability to target a cell and to penetrate a cell's barriers. Chemistry professor Bogdan Dragnea reasoned that it would be possible to get a virus's view of the world by replacing its nucleic acid core with a nanoparticle spy.
"We could have a particle that would be monitored optically or with magnetic resonance imaging, for example, so we could see how viruses move systemically or how viruses bind and deliver their cargo inside a cell," Dragnea said.
Because they can control the size and chemistry of the nanoparticle core, Dragnea's team realized they could also study how the capsid assembles. What determines the number of protein subunits in the capsid, Dragnea wondered. In the brome mosaic virus, for example, 180 protein subunits come together to form the icosahedral shell.
"Since it's a self-assembly process, why couldn't the capsid have 60 subunits or 360 subunits and still have the required icosohedral symmetry," Dragnea asked. This is a matter you can explore chemically, he explained. "Having access to physical characteristics of the core—size, surface charge, and hydrophobicity—we can figure out using models what interactions determine the size of the capsid."
Dragnea and his research team learned that the size of the core, not its charge, determines the size of the capsid. When they used a gold particle with a 5.5-nm diameter as the core, the capsid that formed was made up of 60 protein subunits. When they increased the diameter of the core to 12 nm, the capsid grew to a 180-subunit construct. "That's a very sensitive response to the core volume," Dragnea said. Changes in surface charge of the particle affected only the efficiency of capsid assembly, not its size (Proc. Natl. Acad. Sci. USA 2007, 104, 1354).
From this result, Dragnea believes the size of a virus's genome determines the size of its capsid. "When the protein binds to its nucleic acid core, together they form a polyelectrolyte shell, and those are known to be quite rigid, just like a spherical template. That's how the radius of curvature of the virus is established, by making a rigid shell underneath the capsid surface," he said.
"This has relevance from an applied point of view because if you want to make symmetric, well-defined capsules for transporting cargo, then you want to know how much control you have over the size and the properties of that container," Dragnea continued. "Also, if we find the kinetic bottleneck of virus assembly, we could then look for molecules that bind specifically to that structure" and somehow hinder the formation of viruses.
Dragnea's team has found the size-based assembly mechanism to be general for four different types of viruses—two plant viruses, one bacterial virus, and one animal virus. They also managed to use a range of different nanoparticles as the core, including quantum dots, gold particles, and superparamagnetic iron oxide particles.
The researchers have injected the capsid-covered superparamagnetic iron oxide particles into plants and have monitored the particles' diffusion in the plant vasculature via magnetic resonance imaging. So far, they've learned that it takes only a small concentration of nanoparticles to create an MRI image. "Next, we would like to look at the systemic movement of viruses using magnetic resonance imaging and establish the timescale upon which the viruses move," Dragnea said.
Trading SiO2 Dielectrics for SANDs
CAPITALIZING on the chemistry of self-assembling monolayers, scientists at Northwestern University have developed a new type of gate dielectric material that could enhance the performance of organic and other unconventional electronic devices and could make the devices less expensive and more robust.

Once considered little more than insulation in the architecture of a field-effect transistor, gate dielectric materials have become glamorous in recent years. That's because electronics makers are discovering that these materials can have a profound effect on transistor performance, especially as these devices continue to shrink in size. In fact, chip-making giant Intel announced early this year that it would be swapping its SiO2 dielectrics for hafnium-based materials—a billion-dollar undertaking.
"Everyone has concentrated largely on the semiconductor materials. The dielectric was something that you never worried about," said Tobin J. Marks, a chemistry and materials science professor at Northwestern. "I think it has been sort of an awakening for the community that the dielectric can affect the properties of a transistor so much."
In the structure of a field-effect transistor, the gate dielectric sits between the gate electrode and the semiconductor. It functions as a critical enabling element, stabilizing charge carriers in the channel adjacent to the semiconductor as they pass between the source and drain electrodes.
Traditionally, dielectrics have been made of SiO2, but as this material gets thinner, it tends to leak current. Marks and his research group have been trying to create dielectric materials that are superior to SiO2 and can also be used in organic and other unconventional electronic devices such as flexible electronics printed on plastic.
An ideal gate dielectric material, Marks explained, has a high dielectric constant, is extremely thin, and won't leak current. His research group has recently developed a new type of dielectric material that fits all those criteria.
Called self-assembled nanodielectrics, or SANDs, the materials are composed of self-assembling molecular thin films. "They have dramatic effects on the performance of both organic and inorganic semiconductors," Marks said. They increase the mobility of charge carriers in the semiconductor, greatly reduce the transistor's operating voltage, and suppress trapped charges at the semiconductor-dielectric interface, which make it difficult for the transistor to operate.
Marks's group creates SANDs through a simple dipping and curing process. Monolayers of hydrocarbons or extended π-systems are applied to the gate electrode via the reaction of organochlorosilanes with the electrode's surface hydroxyl groups. A curing process then caps this with a layer of cross-linked siloxane, which can either serve as a base for another layer of organic material or connect the structure to the semiconductor.
A layer made from the extended π-system endows SAND with a very large polarizability, which, Marks explained, helps stabilize charge in the adjacent semiconductor. The cross-linked siloxane that connects SAND to the gate electrode and semiconductor makes the system both electrically and thermally robust. Futhermore, he added, the dipping process used to prepare SANDs allows his group to apply any number of different layers in a manner that's less expensive and simpler to implement than previously reported dielectric deposition processes.
"It works with just about any semiconductor," Marks said, "and it's been useful for improving the performance of all different types of transistors." Working with the company Polyera, which Marks cofounded with Antonio Facchetti, a chemistry professor at Northwestern, these and related materials now are being developed for commercial use.
A Polymer Interface For Prosthetics
PROSTHETIC DEVICES such as artificial limbs, cochlear implants, and pacemakers could soon interface more seamlessly with the body, thanks to the conducting polymer poly(3,4-ethylenedioxythiophene), or PEDOT.
To integrate electronic devices into a biological system, prosthetic makers must find a way to combine two inherently different systems. Devices are typically hard and synthetic and rely upon electrons to transport charge; biological systems, on the other hand, are soft and wet and use ions to transport charge.

"In terms of developing future state-of-the-art prosthetics, you'd like a material that could interact with all the different kinds of biological interfaces-bone, muscle, nerves, and skin," said Sarah M. Richardson-Burns, a senior research fellow in David C. Martin's group at the University of Michigan.
Richardson-Burns and her colleagues have found that PEDOT is able to bridge the electronic and biological worlds, providing a smooth interface from tissue to polymer to electrode. As a class of materials, PEDOT has both excellent durability and remarkable electronic stability, she explained. "If you keep running charge through it over and over again it doesn't degrade. It will maintain its electronic stability."
At first, the researchers began using PEDOT to coat electrodes that were being implanted in tissues. Then it occurred to them that PEDOT could be fully integrated into the tissue and still function as an electrode. By polymerizing PEDOT within the tissue itself, they could minimize the damage done to the tissue by implanted devices.
To polymerize PEDOT, Richardson-Burns and her colleagues apply the 3,4-ethylenedioxythiophene monomer and a small, removable electrode to the tissue and then polymerize the material electrochemically (J. Neural Eng. 2007, 4, L6). "We think the polymer is using the extracellular matrix and the cell membranes as a scaffold for polymerization," she said. The resulting PEDOT-integrated tissue becomes an electrically conductive network that facilitates signal transduction between the ionically conductive tissue and an electrically conductive device.
The in situ polymerization technique has recently been used with cochlear implants in guinea pigs. With current cochlear implants, electrical pulses are used to stimulate auditory nerve endings to create sound sensations, Richardson-Burns said. Forming the PEDOT network directly within the cochlea may allow the prosthetic device to communicate more intimately with the auditory nerves.
Next, they'd like to interface the PEDOT polymer with peripheral nerves to create biointegrated artificial limbs. "Our vision is that you would have an electronic cap that would fit over the end of the limb, and in that cap you would have PEDOT connections to the nerves, muscles, and bone," Richardson-Burns explained. A robotic device that could connect to PEDOT would then be attached to that cap, physically integrating the electronic prosthesis with PEDOT-integrated biological structures.
Richardson-Burns, Martin, and colleagues are currently working to commercialize the PEDOT technology with the start-up company Biotectix, in Quincy, Mass.
Self-Assembling Sacs And Membranes
AS SOPHISTICATED a field as chemistry has become, it's nice to know that chemists can still learn something new through the simple act of mixing two things together and having something completely unexpected happen. That's exactly how Samuel I. Stupp's group discovered a new type of solid membrane.
Stupp, a chemistry and materials science professor at Northwestern University, has been working with a family of compounds called peptide amphiphiles. The molecules feature a hydrocarbon on one end and a peptide, which has been chosen for its self-assembling capabilities and biological activity, on the other end. The compounds, which have shown promise in regenerative medicine, assemble into fibers with the hydrophobic groups at the core and the peptides on the surface.
Curious about how these small self-assembling molecules would interact with large biopolymers, Stupp and his research team decided to see what would happen when they mix an aqueous solution of the positively charged peptide amphiphiles with an aqueous solution of a negatively charged biopolymer.
"We didn't know exactly what was going to happen," Stupp said. On the basis of chemical common sense, Stupp's research group expected that the oppositely charged systems might form a gel or some sort of precipitate. "If you combine two aqueous solutions, particularly if they have opposite charge, you expect that they will mix. That's what every chemist would expect," he said. "Instead we found that there was instant formation of a solid membrane with a high degree of order where the two liquids touched."
Advertisement
There's a dynamic self-assembly process that occurs when the two liquids touch each other, Stupp said. On a molecular level, the biopolymer acts like glue, holding the peptide amphiphile nanofibers in a bundle. "As soon as the polymer gets anywhere near the peptide amphiphile molecules, you see rapid self-assembly," he noted.
Initially, the bundled fibers form strands in the plane of the membrane. As the membrane grows, however, new bundles form perpendicular to the membrane. The process is akin to straining fine spaghetti through a colander. Initially, the pasta lies flat, but over time, strands begin to poke through the colander's holes.
Likewise, the biopolymer pokes through the fibrous mesh. When it does, there's a sea of small molecules waiting to assemble around the biopolymer strands. The membrane will continue to grow in this fashion until one of the reagents is used up or until the membrane becomes too thick for the polymer to diffuse through it, Stupp noted.
The membrane is both mechanically robust and permeable to proteins. It can also close in upon itself to create a biopolymer-filled sac. The sac can be punctured and repaired simply by spot-treating it with a solution of the peptide amphiphile.
Stupp envisions a number of possible applications for these membranes. Sacs made from the membranes could be used to harbor cells for biological experiments. The unusual orientation of the fibers in the membrane could find applications in catalysis or photovoltaics.
"It's very difficult to make something that's microns in thickness and macroscopic in size that has order perpendicular to the plane of membrane," Stupp said. "Here you get this orientation by self-assembly. The system does it on its own."
- A Materials Feast In Boston
- Scientists dig in to sessions on unconventional electronics, nanoparticles, and polymers.
- A Material World
- Images captured during research turned into award-winning art.


Join the conversation
Contact the reporter
Submit a Letter to the Editor for publication
Engage with us on Twitter