Advertisement
Grab your lab coat. Let's get started
Welcome!
Welcome!
Create an account below to get 6 C&EN articles per month, receive newsletters and more - all free.
It seems this is your first time logging in online. Please enter the following information to continue.
As an ACS member you automatically get access to this site. All we need is few more details to create your reading experience.
Not you? Sign in with a different account.
Not you? Sign in with a different account.
ERROR 1
ERROR 1
ERROR 2
ERROR 2
ERROR 2
ERROR 2
ERROR 2
Password and Confirm password must match.
If you have an ACS member number, please enter it here so we can link this account to your membership. (optional)
ERROR 2
ACS values your privacy. By submitting your information, you are gaining access to C&EN and subscribing to our weekly newsletter. We use the information you provide to make your reading experience better, and we will never sell your data to third party members.
Materials
Synthetic Protein Mimics Real Thing
FOLDAMERS: β-Amino acid-based assembly has hallmarks of a true protein
by Stu Borman
February 5, 2007
| A version of this story appeared in
Volume 85, Issue 6

IN THE CLASSIC TALE "Pinocchio," the wood-carver Gepetto made a puppet that turned out to act just like a real boy. In the same spirit of creativity, researchers have created the first nonprotein oligomer that has been verified experimentally to look and act much like a true protein (J. Am. Chem. Soc. 2007, 129, 1532).
Nonproteins that act like proteins have long been sought for their potential use for a range of biomedical applications, including the design of proteinlike therapeutics that wouldn't be degraded by enzymes and could therefore be administered orally.
Chemistry professor Alanna Schepartz and coworkers Douglas S. Daniels, E. James Petersson, and Jade X. Qiu at Yale University have now created such a proteinlike structure, a β-peptide bundle. And they show that it exhibits several hallmarks of real proteins, including exceptional stability, an interior core of water-shunning amino acid side chains, and the ability to fold and unfold reversibly.
Schepartz and coworkers made the structure by synthesizing an oligomer from 12 β-amino acids, which are residues with backbones one carbon atom longer than those of natural α-amino acids. The oligomer forms a helix with three faces, a type not seen in natural (α-amino acid-based) proteins. The researchers designed the helix to display hydrophobic side chains on one face and hydrophilic side chains on the other two.
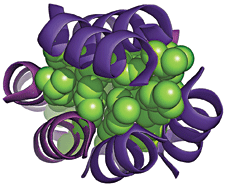
They found that the helices self-assemble into an octameric bundle. When the helices assemble, hydrophilic side chains become arrayed on the outside of the assembly and hydrophobic ones hide inside, similar to the arrangement of side chains in most natural proteins.
The ability to create such "foldamers," peptide- or proteinlike folded structures, has been pursued by several groups. For the most part, they've made helices. The Schepartz group's β-peptide bundle is a higher order structure, an assembly of helices.
Higher order β-peptide foldamers have been made before, but they either weren't fully characterized structurally or weren't as proteinlike as the β-peptide bundle. Extensive complementary interactions between hydrophilic side chains on the bundle's surface help it adopt its discrete higher order structure, whereas earlier β-peptide foldamers tended to aggregate, Schepartz says.
The new JACS paper "describes the first higher order foldamer assembly to be characterized crystallographically," says chemistry professor Samuel H. Gellman of the University of Wisconsin, Madison. The β-peptide bundle "is an important step toward a long-term goal of creating nonnatural molecules with the structure, function, and complexity of proteins," he says.
"It's a major advance," says William DeGrado, professor of biochemistry and biophysics at the University of Pennsylvania. Schepartz and coworkers "have succeeded in a spectacular manner. It's a first move toward the ability to design entirely novel biomimetic polymers from scratch." Demonstrating the ability to tailor-make such foldamers from three-dimensional models could be a focus of future efforts, he says.
The new findings are "what I've been waiting for," comments organic chemistry professor Dieter Seebach of the Swiss Federal Institute of Technology, Zurich. "We can now be sure that there will soon be enzymelike β-peptidic catalysts, which will be stable in a natural environment full of peptidases." That's the type of prediction a creative wood-carver might understand and appreciate.


Join the conversation
Contact the reporter
Submit a Letter to the Editor for publication
Engage with us on Twitter