Advertisement
Grab your lab coat. Let's get started
Welcome!
Welcome!
Create an account below to get 6 C&EN articles per month, receive newsletters and more - all free.
It seems this is your first time logging in online. Please enter the following information to continue.
As an ACS member you automatically get access to this site. All we need is few more details to create your reading experience.
Not you? Sign in with a different account.
Not you? Sign in with a different account.
ERROR 1
ERROR 1
ERROR 2
ERROR 2
ERROR 2
ERROR 2
ERROR 2
Password and Confirm password must match.
If you have an ACS member number, please enter it here so we can link this account to your membership. (optional)
ERROR 2
ACS values your privacy. By submitting your information, you are gaining access to C&EN and subscribing to our weekly newsletter. We use the information you provide to make your reading experience better, and we will never sell your data to third party members.
Materials
Moving Gas Safely
Complexation with low-pressure medium reduces hazards of high-pressure storage, transport
by Mitch Jacoby
January 7, 2008
| A version of this story appeared in
Volume 86, Issue 1
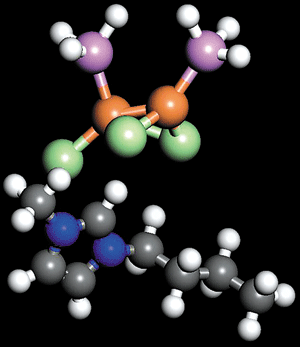
Two phosphine molecules bind to metal atoms in an imidazolium trichorodicuprate compound. Carbon is gray, hydrogen is white, nitrogen is blue, chlorine is green, copper is orange, and phosphorus is purple.
IONIC LIQUIDS CAN HELP MITIGATE some of the hazards of storing and shipping toxic and reactive gases at high pressures, according to a study conducted by researchers at Air Products & Chemicals. The investigation shows that ionic liquids can reversibly take up large quantities of poisonous substances such as phosphine and boron trifluoride by forming chemical complexes with those compounds while retaining the low vapor pressures that are characteristic of ionic liquids (J. Am. Chem. Soc. 2008, 130, 400).
Storing and handling highly toxic compounds at high pressures greatly increases risks of leaks and accidental releases compared with dangerous solids and liquids that have negligibly small vapor pressures. But some gases must be stored at high pressures—for example, those that cannot be liquefied through ordinary industrial processes and compressed liquefied gases that have high vapor pressures even at moderate temperatures. The gas industry traditionally has managed the potential safety hazards associated with such high-pressure gases through judicious use of suitable gas cylinders, valves, and related equipment.

An alternative way to deal with high-pressure safety risks is to sidestep high pressures altogether by packing toxic gases into a medium that can hold large enough quantities of the substances to be practical for industrial use and can readily release the compounds as needed.
That's the approach taken by research chemist Daniel J. Tempel and coworkers at Air Products. Through experimental and computational means, the researchers matched the Lewis acidities and basicities of hazardous gases and ionic liquids into pairs that reversibly form chemical complexes.
In one case, they showed that the toxic gas phosphine, a Lewis base, binds to imidazolium trichorodicuprate ionic liquid, a Lewis acid, in a 2-to-1 molar ratio. Similarly, the group found that the toxic and Lewis acidic gas boron trifluoride binds in equimolar ratios to a basic imidazolium tetrafluoroborate. In both cases, at the maximum gas capacity of the ionic liquids, the pressure remains near 1 atm, which is a tiny fraction of the pressures at which PH3 and BF3 are typically shipped and stored.
Extracting the gas from the ionic liquids requires simply connecting the container in which the complexed material is stored to a vacuum source. That action reverses the complexation process and liberates the reactive gas at modest flow rates.
Daniel F. O'Connell, a business manager with Air Products, notes that the company has recently completed tests in which complexed gases were used with commercial ion-implantation devices to dope semiconductor wafers with boron and phosphorus atoms. O'Connell says the company is currently exploring options for commercializing the gas-storage technology and expects that it will be used for applications in the electronics industry and other areas.
Kenneth R. Seddon, a chemistry professor at Queen's University of Belfast, in Northern Ireland, describes the study as "very important" and notes that it reports on a "a brand-new, green application of ionic liquids." He adds that this ionic-liquid-based method significantly outperforms related efforts to adsorb reactive gases on solids.




Join the conversation
Contact the reporter
Submit a Letter to the Editor for publication
Engage with us on Twitter