Advertisement
Grab your lab coat. Let's get started
Welcome!
Welcome!
Create an account below to get 6 C&EN articles per month, receive newsletters and more - all free.
It seems this is your first time logging in online. Please enter the following information to continue.
As an ACS member you automatically get access to this site. All we need is few more details to create your reading experience.
Not you? Sign in with a different account.
Not you? Sign in with a different account.
ERROR 1
ERROR 1
ERROR 2
ERROR 2
ERROR 2
ERROR 2
ERROR 2
Password and Confirm password must match.
If you have an ACS member number, please enter it here so we can link this account to your membership. (optional)
ERROR 2
ACS values your privacy. By submitting your information, you are gaining access to C&EN and subscribing to our weekly newsletter. We use the information you provide to make your reading experience better, and we will never sell your data to third party members.
Pharmaceuticals
Long Haul From Fundamental Research To Blockbuster Drug
by Mitch Jacoby
March 10, 2008
| A version of this story appeared in
Volume 86, Issue 10
"I thought, 'Wow, that's amazing.' So I sent him [a coworker] back to the lab to do the test again just to be sure."
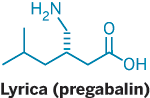
That's how Northwestern's Richard B. Silverman recalls responding nearly 20 years ago to a key piece of news that ultimately led to today's blockbuster nerve pain and epilepsy medication, Lyrica.
Ryszard Andruszkiewicz, then a visiting scholar from Gdansk University of Technology, in Poland, was working with Silverman on a project aimed at finding substances that would inhibit the action of γ-aminobutyric acid aminotransferase (GABA-AT). That enzyme degrades the inhibitory neurotransmitter γ-aminobutyric acid (GABA). As Silverman explains, one cause of convulsions is low brain levels of GABA relative to L-glutamate. The team's goal was to make molecules that would inhibit GABA-AT and raise GABA levels, thereby functioning as an anticonvulsant or antiepilepsy medication.
To that end, Silverman proposed making a series of GABA analogs with various-sized lipophilic substituents (to increase the compounds' chances of crossing the blood-brain barrier) and testing to see which compound best inhibited GABA-AT without inhibiting glutamate decarboxylate, the enzyme that makes GABA.
Andruszkiewicz prepared some 17 compounds and found a correlation between substituent size and the compound's efficiency at inhibiting GABA-AT. The team hoped that if the compounds inhibited one of the enzymes, they wouldn't also inhibit the other enzyme and cancel their beneficial effect on GABA levels.
"To our surprise, we saw just the opposite effect. Not only was the GABA-producing enzyme not inhibited by the GABA analogs we tested, it was activated," Silverman recalls. That's when he sent Andruszkiewicz back to the lab to confirm the unexpected results. He confirmed them—several times for good measure.
Soon drugmaker Parke-Davis agreed to study the compounds' effects in mice. Most were fairly weak anticonvulsants, Silverman says. "But one of them was off the charts." That substance was pregabalin.
From that point on, progress crept along at a snail's pace, Silverman laments. Numerous patent complications and legal barriers and a lengthy clinical-trial process held the promising substance in limbo for nearly 15 aggravating years. During that time, Pfizer acquired Parke-Davis and, eventually, pregabalin came to be the active ingredient now available as Lyrica.
The drug has since been shown effective for treating chronic pain. Silverman has heard from chronic pain sufferers who took the drug and reported enjoying their first eight-hour sleep in 15 years. "It pulls at your heart to think how much misery these people are in," he says. "Knowing that I've done something to help bring relief to people who used to suffer constantly is very rewarding. It makes it all worthwhile."
- Financial Windfall From Lyrica
- Royalty payouts from university-held patents have power to transform chemistry departments
- Drug Discovery
- Long Haul From Fundamental Research To Blockbuster Drug


Join the conversation
Contact the reporter
Submit a Letter to the Editor for publication
Engage with us on Twitter