Advertisement
Grab your lab coat. Let's get started
Welcome!
Welcome!
Create an account below to get 6 C&EN articles per month, receive newsletters and more - all free.
It seems this is your first time logging in online. Please enter the following information to continue.
As an ACS member you automatically get access to this site. All we need is few more details to create your reading experience.
Not you? Sign in with a different account.
Not you? Sign in with a different account.
ERROR 1
ERROR 1
ERROR 2
ERROR 2
ERROR 2
ERROR 2
ERROR 2
Password and Confirm password must match.
If you have an ACS member number, please enter it here so we can link this account to your membership. (optional)
ERROR 2
ACS values your privacy. By submitting your information, you are gaining access to C&EN and subscribing to our weekly newsletter. We use the information you provide to make your reading experience better, and we will never sell your data to third party members.
Synthesis
Frustration Begets Dihydrogen Cleavage
March 17, 2008
| A version of this story appeared in
Volume 86, Issue 11
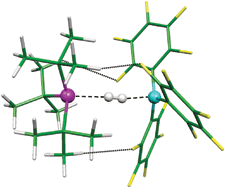
Phosphines and boranes very much like to form Lewis acid-base adducts, but bulky substituent groups can sometimes get in the way and prevent close encounters. Such "frustrated" Lewis pairs, nonetheless, have been shown to facilitate chemical transformations, such as cleaving H2 under mild conditions. A theoretical study led by Imre Pápai and Tibor Soós at the Chemical Research Center of the Hungarian Academy of Science, Budapest, now illustrates a possible mechanism for how such metal-free H2 activation might take place (Angew. Chem. Int. Ed. 2008, 47, 2435). The researchers demonstrated that (tert-butyl)3P and B(C6F5)3 form a flexible, weakly bound complex through C–H···F hydrogen bonds and transient dipole interactions. An H2 molecule may then enter into the phosphine-borane adduct as shown (P is magenta, B is aqua, H is white). As H2 interacts with the phosphorus and boron centers, it becomes polarized such that the electron density shifts away from phosphorus and toward boron. This partial electron transfer weakens the H–H bond, which finally dissociates to form [(tert-butyl)3PH][HB(C6F5)3]. The frustrated phosphine-borane complex, although weakly bound, is a strained system that lowers the activation energy for H2 cleavage, the authors conclude.


Join the conversation
Contact the reporter
Submit a Letter to the Editor for publication
Engage with us on Twitter