Advertisement
Grab your lab coat. Let's get started
Welcome!
Welcome!
Create an account below to get 6 C&EN articles per month, receive newsletters and more - all free.
It seems this is your first time logging in online. Please enter the following information to continue.
As an ACS member you automatically get access to this site. All we need is few more details to create your reading experience.
Not you? Sign in with a different account.
Not you? Sign in with a different account.
ERROR 1
ERROR 1
ERROR 2
ERROR 2
ERROR 2
ERROR 2
ERROR 2
Password and Confirm password must match.
If you have an ACS member number, please enter it here so we can link this account to your membership. (optional)
ERROR 2
ACS values your privacy. By submitting your information, you are gaining access to C&EN and subscribing to our weekly newsletter. We use the information you provide to make your reading experience better, and we will never sell your data to third party members.
Materials
Spotting H2O2
Sensor capitalizes on contrasts in redox chemistry
by Bethany Halford
March 17, 2008
| A version of this story appeared in
Volume 86, Issue 11
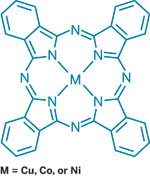
Metallophthalocyanines are key sensing components in a new H2O2 detector.
IN AN EFFORT TO SNIFF OUT peroxide-based explosives, such as those used in the 2005 bombing of the London transit system, scientists have developed a simple, inexpensive sensor that selectively detects hydrogen peroxide vapor (J. Am. Chem. Soc., DOI: 10.1021/ja710324f). In addition to its application in counterterrorism, the device could be used to detect H2O2 in industrial settings, where exposure to the chemical is an important health issue.
The recipe for peroxide-based explosives calls for H2O2 as a precursor, so peroxide bombs usually contain traces of the chemical. The matchbook-sized sensor, invented by a team from the University of California, San Diego, led by William C. Trogler, Andrew C. Kummel, and Ivan K. Schuller, can detect H2O2 vapor in the parts-per-billion range. According to the researchers, the cheap, compact device improves upon standard methods of H2O2 detection, which rely on complex, expensive instruments to do the job.
The device employs thin films of metal phthalocyanines as its key sensing components. These films are chemiresistors—their conductivity varies depending on their exposure to different chemicals. "For metal phthalocyanines, oxidants normally introduce an increase in current," whereas reducing agents have the opposite effect, Trogler explains.
In the presence of the oxidant H2O2, however, metal phthalocyanines behave differently. Cobalt phthalocyanine shows a decrease in current, but other phthalocyanines with metals, such as copper or nickel, show an increase in current. A sensor array with both cobalt phthalocyanine and copper phthalocyanine therefore displays a unique signature for H2O2.
Joseph Wang, a chemical engineering professor at Arizona State University, calls the work "a very elegant and effective approach for detecting hydrogen peroxide vapor." He adds that the "ability to tailor the response via a judicious choice of the specific metal center of the phthalocyanine holds great promise for a wide range of monitoring applications."



Join the conversation
Contact the reporter
Submit a Letter to the Editor for publication
Engage with us on Twitter