Advertisement
Grab your lab coat. Let's get started
Welcome!
Welcome!
Create an account below to get 6 C&EN articles per month, receive newsletters and more - all free.
It seems this is your first time logging in online. Please enter the following information to continue.
As an ACS member you automatically get access to this site. All we need is few more details to create your reading experience.
Not you? Sign in with a different account.
Not you? Sign in with a different account.
ERROR 1
ERROR 1
ERROR 2
ERROR 2
ERROR 2
ERROR 2
ERROR 2
Password and Confirm password must match.
If you have an ACS member number, please enter it here so we can link this account to your membership. (optional)
ERROR 2
ACS values your privacy. By submitting your information, you are gaining access to C&EN and subscribing to our weekly newsletter. We use the information you provide to make your reading experience better, and we will never sell your data to third party members.
Biological Chemistry
New Twist On Amyloids
Handedness hints at how peptides organize
by Carmen Drahl
March 31, 2008
| A version of this story appeared in
Volume 86, Issue 13
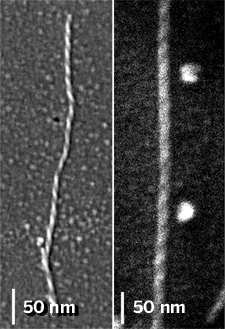
AGGREGATES OF DIFFERENT types of amyloid-forming peptides have different chiralities, a new study shows (J. Am. Chem. Soc., DOI: 10.1021/ja800328y). The finding gives clues about how these different peptides assemble and raises questions about the relationship between amino acid sequence, handedness, and amyloid—related ailments such as Alzheimer's disease.
Amyloids are complex protein aggregates with multiple levels of organization. Noa Rubin, Emanuel Perugia, Michal Goldschmidt, Mati Fridkin, and Lia Addadi of the Weizmann Institute of Science in Rehovot, Israel, have now used scanning electron microscopy (SEM) to learn more about one aspect of this hierarchy-amyloid chirality.

The researchers examined three different amyloid fibers, each made from multiple copies of a disease-associated peptide segment from a different protein. They found that fibers made from a serum amyloid A peptide (SAA) possess a right-handed twist, and fibers from the other two peptides twist leftward.
Amyloid fibers from different proteins, including SAA, are thought to have a common molecular organization called a cross-β structure, the authors write in their paper. They say that it is unclear whether this structure is always made from β-sheets, a common type of peptide conformation, or other peptide folds.
β-Sheets made of naturally occurring amino acids have a chiral signature that Addadi and coworkers looked for in their experiments—they twist leftward. "The left-handedness has to do with the chirality of the amino acids that are in the sequence," Addadi explains. Any fiber that forms from an assembly of multiple β-sheets will have a detectable left-handed twist, she says.
The research team's SEM images showed, however, that SAA fibers are consistently right-handed. In addition, fibers they made from a synthetic peptide that is the mirror image of SAA had a left-handed twist. Addadi says this indicates that SAA's cross-β structure is not made from β-sheets. The team next hopes to identify the underlying SAA structure in the aggregates.
This work "shows that while amyloid fibril formation is the fate of many proteins and peptides, there are undoubtedly distinct ways in which amyloid fibrils form," says Bart E. Kahr of the University of Washington, Seattle, who studies aggregate formation.
The distinct ways in which amyloid fibrils form are not surprising, the authors write in their paper. The relationships between molecular structure and supramolecular aggregate structure in many biomolecules, such as DNA, are complex, they add.
The Weizmann team's work falls in line with those observations, says Samuel I. Stupp of Northwestern University, who studies self-assembled structures. "Direct observations of this complexity in molecules so relevant to human disease raise questions about the possible pathological influence of suprastructures with chiralities that differ from those prevalent in biology," Stupp says.


Join the conversation
Contact the reporter
Submit a Letter to the Editor for publication
Engage with us on Twitter