Advertisement
Grab your lab coat. Let's get started
Welcome!
Welcome!
Create an account below to get 6 C&EN articles per month, receive newsletters and more - all free.
It seems this is your first time logging in online. Please enter the following information to continue.
As an ACS member you automatically get access to this site. All we need is few more details to create your reading experience.
Not you? Sign in with a different account.
Not you? Sign in with a different account.
ERROR 1
ERROR 1
ERROR 2
ERROR 2
ERROR 2
ERROR 2
ERROR 2
Password and Confirm password must match.
If you have an ACS member number, please enter it here so we can link this account to your membership. (optional)
ERROR 2
ACS values your privacy. By submitting your information, you are gaining access to C&EN and subscribing to our weekly newsletter. We use the information you provide to make your reading experience better, and we will never sell your data to third party members.
Physical Chemistry
Benzene Stand-Ins
Crystal structures indicate that rings containing a boron-nitrogen bond are aromatic
by Carmen Drahl
June 16, 2008
| A version of this story appeared in
Volume 86, Issue 24

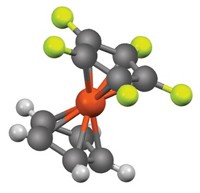
WITH A SYSTEMATIC SERIES of X-ray crystal structures, chemist Shih-Yuan Liu and coworkers at the University of Oregon, Eugene, provide evidence that a family of six-membered rings featuring a boron-nitrogen bond has a benzenelike nature (J. Am. Chem. Soc. 2008, 130, 7250). The compounds, 1,2-dihydro-1,2-azaborines, feature bonding characteristics and other properties that could make them valuable substitutes for benzenes in biomedical and materials research.
Benzene is the quintessential aromatic compound, stabilized by virtue of delocalized electrons freely moving about its circular array of atoms. Liu's work indicates that 1,2-dihydro-1,2-azaborines, related to benzene through substitution of one of the ring's bonds with a B–N bond, share that special stability.
Liu and coworkers synthesized a derivative of 1,2-dihydro-1,2-azaborine with substituents at the boron and nitrogen atoms. They also made four reference heterocycles designed to be nonaromatic. They then obtained X-ray crystal structures of all five compounds. Compared to the reference compounds, the purported benzene surrogate showed clear signs of the electron delocalization that is emblematic of aromatic compounds, such as a more planar six-membered ring with more homogeneous bond lengths.
The work "is extremely rigorous and provides unambiguous evidence for the aromatic character of 1,2-dihydro-1,2-azaborines," says Francois P. Gabbai, a boron chemistry expert at Texas A&M University.
"This is a thorough and beautifully simple study," adds Warren E. Piers of the University of Calgary, in Alberta, who has utilized the B N bond's increased ionic character relative to an all-carbon bond to make polycyclic materials.
The results, Liu cautions, are just one piece of evidence for aromaticity and should be taken together with studies conducted by other teams.
Nonetheless, the findings enrich scientists' understanding of aromaticity, a key concept of organic chemistry, says Todd B. Marder of Durham University, in England, who studies boron compounds. "This is the kind of fundamental chemistry that will no doubt find its way into textbooks," he says.



Join the conversation
Contact the reporter
Submit a Letter to the Editor for publication
Engage with us on Twitter