Advertisement
Grab your lab coat. Let's get started
Welcome!
Welcome!
Create an account below to get 6 C&EN articles per month, receive newsletters and more - all free.
It seems this is your first time logging in online. Please enter the following information to continue.
As an ACS member you automatically get access to this site. All we need is few more details to create your reading experience.
Not you? Sign in with a different account.
Not you? Sign in with a different account.
ERROR 1
ERROR 1
ERROR 2
ERROR 2
ERROR 2
ERROR 2
ERROR 2
Password and Confirm password must match.
If you have an ACS member number, please enter it here so we can link this account to your membership. (optional)
ERROR 2
ACS values your privacy. By submitting your information, you are gaining access to C&EN and subscribing to our weekly newsletter. We use the information you provide to make your reading experience better, and we will never sell your data to third party members.
Analytical Chemistry
Electron Microscopy For Chemists
Advances in imaging and elemental analysis move TEM toward the realm of analytical chemistry
by Mitch Jacoby
June 23, 2008
| A version of this story appeared in
Volume 86, Issue 25
CRUISING AT about half the speed of light, tightly focused beams of electrons pass through thin slices of materials and carry away subtle information about the substance's structure and composition. Commonly known as transmission electron microscopy (TEM), this high-energy method has been used for decades to deduce the positions of rows of atoms in solids, often with angstrom-level resolution.
Traditionally, TEM's strong suit has been spotting the odd men out—out-of-place atoms and other types of crystal lattice defects. But scientists are increasingly pushing the limits of TEM to extract chemical information from microscopy samples, and they are doing so with ever finer spatial resolution.
"Angstrom by angstrom, TEM is moving toward imaging individual lightweight atoms," such as those making up organic molecules, says Laurence D. Marks, a microscopist and professor of materials science at Northwestern University. At the same time, the field is progressing toward atomic-scale chemical analysis of lightweight atoms, as new instrument designs enable microscopy and spectroscopy to be carried out simultaneously.
The challenge in observing individual atoms with an electron microscope is that single atoms—in particular, lightweight atoms such as hydrogen and carbon—don't scatter the microscope's electron beam very much at all. Yet it is the scattered beam, which chemists might refer to as the analytical signal, that contains information about the atom's position and identity. "In the past, that information has usually been buried in the noise," Marks says.
Recently, however, improvements in instrument design, some of which Marks characterizes as "revolutionary," have caused the signal-to-noise ratios in electron microscopy to soar, and researchers have lost no time in exploiting these enhanced capabilities.
At state-of-the art TEM facilities, microscopists have mapped out the positions and chemical identities of the atoms in layered metal oxides, for example. In other analyses, they've pinpointed with angstrom resolution the locations of single atomic impurities in nanowires. Some researchers have tapped TEM's unique capabilities to render three-dimensional maps of nanoparticles dispersed on the interior surfaces of porous materials. And still other scientists have used TEM to record videos showing individual organic molecules undergoing subtle structural changes.
One of the design improvements that microscopists credit for bolstering TEM's signal-to-noise ratio is found way up at the top of the microscope. Today's cold field-emission electron sources significantly outshine conventional hot filaments, the electron sources that have been used for decades to generate the instrument's electron beam. The new sources, which can provide a 10-fold increase in electron current relative to older ones, consist of a fine metal tip that emits electrons when subjected to a powerful electric field.
"A brighter source means more electron current is available for imaging samples," Marks explains. He adds that other improvements, such as those made in dampening tiny mechanical vibrations in the sample stage (holder) and throughout the instrument, further boost the resolving power of modern microscopes.
PERHAPS THE most critical advance, one that's generating quite a buzz in the TEM community, resides with the lenses that focus the electron beam as it travels along the microscope column. Traditional electron microscope lenses suffer from a focusing problem known as spherical aberration. This common shortcoming limits the instrument's resolution and distorts images in a way that is especially noticeable in atomic-scale imaging studies. Within the past few years, however, manufacturers have begun commercializing aberration correctors, which are made up of a number of computer-controlled quadrupole and octopole electron lenses and other types of focusing optics.
"These aberration correctors are rather like the special lenses that were designed to correct the vision of the Hubble Space Telescope," says John Silcox, a professor of applied and engineering physics at Cornell University. The microscope fix was a long time in the making, with roots extending to the 1940s, Silcox says, but a number of factors delayed its implementation. For example, instrument manufacturers have only recently had ready access to the computing power needed to design and build the aberration-correcting units.

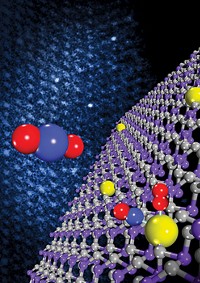
JUST A HANDFUL of microscopes nowadays employ the new technology. One such instrument has enabled Silcox and fellow Cornell applied physics professor David A. Muller and their coworkers to probe materials with extraordinary resolution. Earlier this year, the researchers put their new instrument, a scanning transmission electron microscope made by Kirkland, Wash.-based company Nion, through the paces by imaging and analyzing a layered oxide material, La0.7Sr0.3MnO3/SrTiO3.
Working with Ondrej L. Krivanek, Nion's cofounder who designed the aberration corrector, the Cornell team scanned the electron beam across the oxide crystal and then constructed 2-D maps showing the positions and chemical identities of the atoms in the material. They also showed that one of the elements, titanium, exists in two distinct chemical environments within the sample (Science 2008, 319, 1073).
The chemical identity information comes from electron energy-loss spectroscopy (EELS) measurements that the group makes in tandem with the imaging part of the experiment. In a typical TEM study, electrons race through the microscope with up to a few hundred thousand electron volts of energy. As the electrons zip through the sample material, they lose a bit of energy through a variety of interactions with atoms in the specimen.
In one of those types of events, the speeding electrons ionize sample atoms by imparting enough energy to the sample's inner-shell electrons to eject them. The energy loss, which is element-specific and readily measured during TEM imaging, uniquely identifies the atoms sitting in the beam path. Some microscopists also derive chemical information from a related process that leads to emission of X-ray photons with element-specific energies.
In the Cornell study, the team imaged the material at atomic resolution and simultaneously collected lanthanum, manganese, and titanium EELS data. Then they color-coded the results to represent each type of element. One of the key observations was that diffusion occurred at the interfaces. Specifically, at the boundaries between layers of lanthanum strontium manganese oxide (LSMO) and strontium titanate (STO), some intermixing occurred between manganese and titanium atoms, represented as red and blue, respectively. The intermingling among the elements showed up as atom-wide lines of purple at the interfaces.
Furthermore, the group found that titanium's EELS signature depended on the chemical environment in which the metal atoms were located. As such, they observed marked differences between titanium atoms in the LSMO and STO layers.
The LSMO/STO material, which was custom synthesized by team members in Japan, served primarily as a TEM test specimen. Yet the material is expected to have "rather interesting magnetic and electronic properties," Muller says. But in order for that kind of a layered material to exhibit tailored properties, the interfaces need to be atomically sharp. In this case, they're not. As one outcome of the study, according to Muller, the team learned that there's room for improvement when it comes to preparing these complex layered materials.
"When you're trying to grow nanoscale structures for use in electronic devices, knowing whether the atoms stay put or move around from layer to layer is really important," Muller stresses. The team sees this type of analysis, which Silcox refers to as "materials pathology," as a key application of their new TEM methodology.

Leslie J. Allen, a physics professor at the University of Melbourne, in Australia, sees it that way too. Simultaneous measurement of structural and chemical information at the atomic scale, as demonstrated by the Cornell study, provides fundamental insights into the connection between a material's form and its function or properties, Allen wrote recently in Nature Nanotechnology (2008, 3, 255).
Allen notes that this scanning TEM methodology should be well suited to detailed investigations of carbon nanotubes, semiconductor nanostructures, and catalytic nanoparticles. The TEM technique should also be useful for locating and analyzing single impurity atoms in quantum devices, he adds.
THAT'S EXACTLY what a team of U.S. and U.K.-based researchers did recently with the benefit of aberration-corrected TEM and a complementary method known as field-emission microscopy. The group, which includes Northwestern University assistant professor of physics Lincoln J. Lauhon, Andrew L. Bleloch of the University of Liverpool, and coworkers, analyzed the purity of silicon nanowires grown via the so-called vapor-liquid-solid method. Because the method makes use of gold nanoparticles to catalyze wire growth, researchers have been concerned that if even tiny numbers of gold atoms infiltrate the wires, their properties will be altered to the detriment of future electronic devices constructed from the nanowires.
Those concerns weren't unfounded. In their microscopy study, the group detected single gold atoms in the silicon nanowires and noted that gold's concentration was greater than what would be predicted from the metal's bulk solubility in silicon (Nat. Nanotechnol. 2008, 3, 168).

Analyzing materials with TEM methods is also crucial to the research efforts of inorganic chemist Krijn P. de Jong at Utrecht University, in the Netherlands. TEM is unique among the many analytical methods his lab uses to characterize catalysts, de Jong says. Of special importance is TEM's ability to probe 3-D nanostructured materials, such as zeolites and other highly porous materials that are often used as catalyst supports. By way of comparison, de Jong notes that scanning tunneling microscopy and atomic force microscopy, although powerful tools in their own right, are limited to analyzing a material's external surface. Porous catalyst supports, however, tend to have far more internal surface area than external surface area. As such, TEM's ability to access internal surfaces makes that microscopy method the one of choice in the Utrecht catalysis studies.
In one of the group's investigations, de Jong, Heiner Friedrich, and coworkers used TEM-based electron tomography to map the 3-D positions and analyze other properties of some 300 catalytic nickel oxide nanoparticles in individual pores of SBA-15, a porous silica material (J. Amer. Chem. Soc. 2007, 129, 10249). Related catalysts are used in the hydrogenation of edible vegetable oils to improve the product's flavor, stability, and other properties.
Currently, the group is conducting similar electron tomography studies on cobalt oxide nanoparticles to determine their 3-D arrangement within a carbon nanofiber support material. Those particles are similar to catalysts used in making synthetic fuels via the Fischer-Tropsch process.
"Now that we have a complete geometric description of a single pore loaded with nanoparticles, we can analyze the zigzagging path required for a molecule to follow from pore entrance to pore exit," de Jong says. That level of detail can be helpful in understanding catalytic reaction mechanisms at the nanoscale. Until recently, he says, "having that kind of information was unthinkable."
In the future, de Jong would like to go further and use this sort of detailed information to prepare single pores with well-placed catalyst particles of various types. His dream is to design individual pores that facilitate multiple chemical reactions in a predetermined sequence.
Another current thrust in TEM-based research focuses on developing methods for capturing images and videos of single organic molecules in motion. Last year, a team led by Eiichi Nakamura, a chemistry professor at the University of Tokyo, reported on a vapor-phase-based method for confining single carborane molecules inside a carbon nanotube and imaging them with near atomic resolution. The team observed a carborane molecule shuttling along the length of the tube and undergoing conformational changes (C&EN, Feb. 26, 2007, page 11).
MORE RECENTLY, the team developed a solution-based procedure for introducing larger molecules—this time aminopyrene derivatives—into nanotubes. They found that the molecules' fullerene moieties anchor the compounds and fix their positions within the nanotubes long enough for the molecules' conformational and rotational motions to be imaged (Chem. Lett. 2007, 36, 1208).
And earlier this month, Nakamura and coworkers published TEM images and videos that depict a still larger molecule, a biotinylated triamide, undergoing a variety of shape-altering conformational changes (J. Amer. Chem. Soc., DOI: 10.1021/ja8022708).
In the latest study, the triamide molecule was attached via an amide linkage to the exterior of a carbon-nanotube-like structure in order to remove the conformational constraints imposed by encapsulating molecules within nanotubes. With plenty of room to flex its bonds outside the nanotube, the molecule is free to adopt any of 100 million or so conformations, Nakamura says.
Advertisement
So to aid interpretation of the TEM data, the team calculated the molecular conformation most likely to represent the configuration of the molecule as imaged at specific times during the experiment. They then carried out simulations to show how a real triamide molecule caught in those calculated configurations would appear in TEM images and videos. They report that the simulated and measured images agree closely.
Along the same lines, Zheng Liu, Kazu Suenaga, Sumio Iijima, and coworkers at the National Institute of Advanced Industrial Science & Technology in Japan used TEM techniques to spy on a molecule found in photoreceptor cells as the molecule underwent shape changes. Specifically, the team published TEM images last year depicting the cis-trans isomerization of retinal (Nat. Nanotechnol. 2007, 2, 422). The images represent the first direct observations by any method of a conjugated carbon chain exhibiting shape-changing behavior.

So where else might scientists take TEM? The University of Melbourne's Allen says the next big step in chemical mapping is moving TEM's 2-D projections into the third dimension. The strategy there, he explains, is to change the position of the focal point in the vertical (electron beam) direction while continuing to scan in the other two horizontal directions. Allen and others are now working toward that goal.
Meanwhile, in the world of single-molecule TEM analysis, Nakamura is pushing his methods toward the study of larger and larger molecules. Optimistic because of his group's recent successes, Nakamura says, "We have considerable faith that our TEM method will be useful for imaging small proteins and their motions."
For decades, transmission electron microscopy has been a tool favored by physicists, metallurgists, and materials scientists. Chemists haven't typically found much use for it. But with continued advances in atomic-resolution structural and chemical analysis, TEM may someday soon become a chemist's analytical tool of choice.






Join the conversation
Contact the reporter
Submit a Letter to the Editor for publication
Engage with us on Twitter