Advertisement
Grab your lab coat. Let's get started
Welcome!
Welcome!
Create an account below to get 6 C&EN articles per month, receive newsletters and more - all free.
It seems this is your first time logging in online. Please enter the following information to continue.
As an ACS member you automatically get access to this site. All we need is few more details to create your reading experience.
Not you? Sign in with a different account.
Not you? Sign in with a different account.
ERROR 1
ERROR 1
ERROR 2
ERROR 2
ERROR 2
ERROR 2
ERROR 2
Password and Confirm password must match.
If you have an ACS member number, please enter it here so we can link this account to your membership. (optional)
ERROR 2
ACS values your privacy. By submitting your information, you are gaining access to C&EN and subscribing to our weekly newsletter. We use the information you provide to make your reading experience better, and we will never sell your data to third party members.
Biological Chemistry
Antibiotics From The Deep
January 28, 2008
| A version of this story appeared in
Volume 86, Issue 4
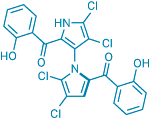
A veritable treasure trove of medicinally relevant compounds has once again been found by plumbing the oceans' depths. William Fenical and coworkers at Scripps Institution of Oceanography have identified an unusual pair of antibiotics isolated from bacteria that inhabit ocean sediments. The compounds, dubbed marinopyrrole A (shown) and B, possess an N,C2-linked bispyrrole motif that's never been observed in natural products (Org. Lett., DOI: 10.1021/ol702952n). Postdoc Chambers C. Hughes isolated the chiral molecules as single atropo-enantiomers, suggesting that their biosynthesis involves a critical enzyme-mediated pyrrole coupling. Fenical and his team were able to isomerize marinopyrrole A to its nonnatural atropo-enantiomer by heating it. This enantiomer, along with the natural marinopyrroles, exhibits promising antimicrobial activity against methicillin-resistant Staphylococcus aureus. By focusing a synthetic effort on the marinopyrroles' novel bispyrrole structure, Fenical says, chemists might discover new compounds for fighting drug-resistant infections.




Join the conversation
Contact the reporter
Submit a Letter to the Editor for publication
Engage with us on Twitter