Advertisement
Grab your lab coat. Let's get started
Welcome!
Welcome!
Create an account below to get 6 C&EN articles per month, receive newsletters and more - all free.
It seems this is your first time logging in online. Please enter the following information to continue.
As an ACS member you automatically get access to this site. All we need is few more details to create your reading experience.
Not you? Sign in with a different account.
Not you? Sign in with a different account.
ERROR 1
ERROR 1
ERROR 2
ERROR 2
ERROR 2
ERROR 2
ERROR 2
Password and Confirm password must match.
If you have an ACS member number, please enter it here so we can link this account to your membership. (optional)
ERROR 2
ACS values your privacy. By submitting your information, you are gaining access to C&EN and subscribing to our weekly newsletter. We use the information you provide to make your reading experience better, and we will never sell your data to third party members.
Synthesis
Selective Pathway To Small-Ring Aldehydes
by Stephen K. Ritter
October 6, 2008
| A version of this story appeared in
Volume 86, Issue 40
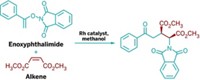
Formylcyclopropanes—three-membered rings with a pendant aldehyde substituent—are some of the most sought-after building blocks in synthetic chemistry. The compounds have the benefit of being biologically active, and the aldehyde functionality can be readily transformed into other useful groups. There are a variety of established synthetic approaches to prepare these compounds, including cycloaddition reactions or by starting with substituted cyclopropanes, but a new approach developed by William M. Sherrill and Michael Rubin of the University of Kansas is the first reported catalytic diastereo- and enantioselective hydroformylation of cyclopropenes to make chiral formylcyclopropanes (J. Am. Chem. Soc., DOI: 10.1021/ja805059f). The researchers reacted various substituted cyclopropenes with synthesis gas (CO and H2) under mild conditions (60 °C and 150 psi) in toluene. The reactions in general required only small amounts of the standard hydroformylation precatalyst, Rh(acetylacetonate)(CO)2), which was coupled with a rigid phosphinoferrocene ligand in situ to impart diastereoselectivity or chiral phosphine ligands to enhance enantioselectivity. The method represents "a convenient, atom-economic approach toward optically active cyclopropylcarboxaldehydes from readily available prochiral cyclopropenes," the researchers write.



Join the conversation
Contact the reporter
Submit a Letter to the Editor for publication
Engage with us on Twitter