Advertisement
Grab your lab coat. Let's get started
Welcome!
Welcome!
Create an account below to get 6 C&EN articles per month, receive newsletters and more - all free.
It seems this is your first time logging in online. Please enter the following information to continue.
As an ACS member you automatically get access to this site. All we need is few more details to create your reading experience.
Not you? Sign in with a different account.
Not you? Sign in with a different account.
ERROR 1
ERROR 1
ERROR 2
ERROR 2
ERROR 2
ERROR 2
ERROR 2
Password and Confirm password must match.
If you have an ACS member number, please enter it here so we can link this account to your membership. (optional)
ERROR 2
ACS values your privacy. By submitting your information, you are gaining access to C&EN and subscribing to our weekly newsletter. We use the information you provide to make your reading experience better, and we will never sell your data to third party members.
Synthesis
Meltable, Moldable Nitrate Explosive
by Elizabeth K. Wilson
October 20, 2008
| A version of this story appeared in
Volume 86, Issue 42
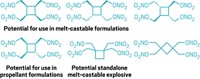
Chemists have synthesized a novel nitrate ester explosive that has the rare and desirable property of being a solid with a melting point low enough so that it can be poured into molds for casting into different shapes (Angew. Chem. Int. Ed. 2008, 47, 8306). David E. Chavez and colleagues at Los Alamos National Laboratory report that the compound, which sports four nitrate ester groups (ONO2) and two nitro groups (NO2), melts at 85 ºC, well before its decomposition point of 141 ºC. The nitrate ester family of explosives got started with the discovery of nitroglycerin in 1846. However, almost all of the compounds are liquids at ambient temperatures. Nitrocellulose is one example of a solid nitrate ester, as is pentaerythritol tetranitrate (PETN). But PETN has a high melting point, requiring that it be pressed into desired shapes. The new tetranitrate ester shares PETN's sensitivity to friction and sparks, and it has explosive capabilities like those of the compound HMX, a cyclic tetranitro compound that is one of the highest performing explosives.





Join the conversation
Contact the reporter
Submit a Letter to the Editor for publication
Engage with us on Twitter