Advertisement
Grab your lab coat. Let's get started
Welcome!
Welcome!
Create an account below to get 6 C&EN articles per month, receive newsletters and more - all free.
It seems this is your first time logging in online. Please enter the following information to continue.
As an ACS member you automatically get access to this site. All we need is few more details to create your reading experience.
Not you? Sign in with a different account.
Not you? Sign in with a different account.
ERROR 1
ERROR 1
ERROR 2
ERROR 2
ERROR 2
ERROR 2
ERROR 2
Password and Confirm password must match.
If you have an ACS member number, please enter it here so we can link this account to your membership. (optional)
ERROR 2
ACS values your privacy. By submitting your information, you are gaining access to C&EN and subscribing to our weekly newsletter. We use the information you provide to make your reading experience better, and we will never sell your data to third party members.
Synthesis
Carbon Nitride Makes Hydrogen Cheaply
Carbon nitride, C3N4, is an inexpensive, stable, and metal-free photocatalyst for producing hydrogen from water
by Jyllian N. Kemsley
November 17, 2008
| A version of this story appeared in
Volume 86, Issue 46

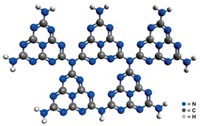
Carbon nitride is an inexpensive, stable, and metal-free photocatalyst for producing hydrogen from water, reports a group led by Xinchen Wang of the International Joint Laboratory, a joint venture between the Max Planck Institute of Colloids & Interfaces, in Potsdam, Germany, and Fuzhou University, in China, and Kazunari Domen of the University of Tokyo (Nat. Mater., DOI: 10.1038/nmat2317). Typical H2-producing catalysts either require precious metals such as platinum or ruthenium or, in the case of synthetic polymer semiconductors, ultraviolet light. Wang, Domen, and colleagues found that "graphitic" carbon nitride—layers of C3N4 sheets—can produce H2 in visible light without metal additives when triethanolamine is present as a sacrificial electron donor. Density functional computations indicate that the material likely oxidizes H2O to O2 at the nitrogen atoms and reduces H+ to H2 at the carbon atoms. The unoptimized carbon nitride system produced up to 4µmol of H2 per hour, roughly an order of magnitude less than photocatalysts that use precious metals. The result "opens new vistas for the search of energy production schemes" using commonly available materials, the authors write.





Join the conversation
Contact the reporter
Submit a Letter to the Editor for publication
Engage with us on Twitter