Advertisement
Grab your lab coat. Let's get started
Welcome!
Welcome!
Create an account below to get 6 C&EN articles per month, receive newsletters and more - all free.
It seems this is your first time logging in online. Please enter the following information to continue.
As an ACS member you automatically get access to this site. All we need is few more details to create your reading experience.
Not you? Sign in with a different account.
Not you? Sign in with a different account.
ERROR 1
ERROR 1
ERROR 2
ERROR 2
ERROR 2
ERROR 2
ERROR 2
Password and Confirm password must match.
If you have an ACS member number, please enter it here so we can link this account to your membership. (optional)
ERROR 2
ACS values your privacy. By submitting your information, you are gaining access to C&EN and subscribing to our weekly newsletter. We use the information you provide to make your reading experience better, and we will never sell your data to third party members.
Synthesis
Different Metal, Different Isomer
Simply choosing a ruthenium or a palladium catalyst provides a novel means of diastereocontrol when making complex bicyclic compounds
by Stephen K. Ritter
November 17, 2008
| A version of this story appeared in
Volume 86, Issue 46
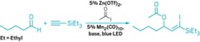
Exquisite control over the stereoselective synthesis of bicyclic compounds is possible simply by choosing one catalyst metal over another, report Barry M. Trost, Eric M. Ferreira, and Alicia C. Gutierrez of Stanford University (J. Am. Chem. Soc., DOI: 10.1021/ja8078835). Trost's group introduced the metal-catalyzed enyne cycloisomerization reaction in 1985 as a strategy for synthesizing complex organic molecules. Follow-up studies have expanded the scope of the reaction and provided insight into the mechanism. In the latest rendition of the reaction, Trost and coworkers converted an alkyne-substituted cyclohexene into a bicyclic compound. The trans stereoisomer forms when a ruthenium catalyst is used, and the cis version forms when a palladium catalyst is used (shown). The difference plays out in one of the proposed intermediates: Both metals form a cyclic complex via coordination to the alkene and alkyne bonds of the substrate, but ruthenium uses the carbonyl oxygen of one methyl ester group to temporarily steady itself, whereas palladium does not. The result is that the carbonyl directs formation of the trans isomer, providing a novel means of diastereocontrol.



Join the conversation
Contact the reporter
Submit a Letter to the Editor for publication
Engage with us on Twitter