Advertisement
Grab your lab coat. Let's get started
Welcome!
Welcome!
Create an account below to get 6 C&EN articles per month, receive newsletters and more - all free.
It seems this is your first time logging in online. Please enter the following information to continue.
As an ACS member you automatically get access to this site. All we need is few more details to create your reading experience.
Not you? Sign in with a different account.
Not you? Sign in with a different account.
ERROR 1
ERROR 1
ERROR 2
ERROR 2
ERROR 2
ERROR 2
ERROR 2
Password and Confirm password must match.
If you have an ACS member number, please enter it here so we can link this account to your membership. (optional)
ERROR 2
ACS values your privacy. By submitting your information, you are gaining access to C&EN and subscribing to our weekly newsletter. We use the information you provide to make your reading experience better, and we will never sell your data to third party members.
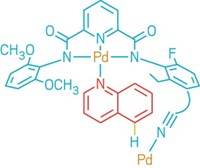
Synthesis
Functionalizing Silicon
Classic organic reaction modifies semiconductors
by Mitch Jacoby
November 24, 2008
| A version of this story appeared in
Volume 86, Issue 47

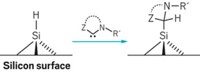
BY DEVELOPING A WAY to apply a common chemical reaction to silicon surfaces, researchers in Delaware have broadened techniques available for modifying semiconductors with organic molecules. The work details a procedure for carrying out surface-dehydrative condensation reactions using standard equipment and mild conditions (J. Am. Chem. Soc., DOI: 10.1021/ja802645t).
An overarching strategy for advancing the emerging field of molecular electronics calls for marrying organic chemistry—a discipline with a huge number of well-studied chemical transformations—with semiconductors, the platform on which microelectronics is built.
Working toward that goal in recent years, scientists have devised surface-chemistry analogs of classic organic processes, including Diels-Alder, Grignard-type, and cycloaddition reactions. The Delaware team has now extended that list.
Timothy R. Leftwich, Mark R. Madachik, and Andrew V. Teplyakov have shown that silicon wafers with hydrogen-terminated surfaces are readily functionalized via dehydrative cyclocondensation reactions.
Demonstrating that process, the group showed that nitrobenzene reacts to form a surface-bound nitrosobenzene adduct. The process combines two surface hydrogens with an oxygen from the nitro group to eliminate a molecule of water. Using surface spectroscopy methods, the team verified that nitrosobenzene is attached to the surface via one O–Si bond and one N–Si bond and that the C–N bond and phenyl ring remain intact.
Teplyakov points out that in contrast to other organic surface reactions, the new process can be carried out with standard laboratory equipment and does not require radical initiators, photochemical steps, or chemical solutions of modifier compounds, which are possible sources of contamination.
"This work describes an interesting and potentially useful addition to the toolbox of reactions for functionalizing silicon surfaces," says Kate Queeney, a surface chemist at Smith College, in Northampton, Mass. Although the reaction has the drawback of forming adducts that adsorb to the surface in more than one way, it provides a way to introduce functional groups that are not cleanly accessible by other routes, she adds.


Join the conversation
Contact the reporter
Submit a Letter to the Editor for publication
Engage with us on Twitter