Advertisement
Grab your lab coat. Let's get started
Welcome!
Welcome!
Create an account below to get 6 C&EN articles per month, receive newsletters and more - all free.
It seems this is your first time logging in online. Please enter the following information to continue.
As an ACS member you automatically get access to this site. All we need is few more details to create your reading experience.
Not you? Sign in with a different account.
Not you? Sign in with a different account.
ERROR 1
ERROR 1
ERROR 2
ERROR 2
ERROR 2
ERROR 2
ERROR 2
Password and Confirm password must match.
If you have an ACS member number, please enter it here so we can link this account to your membership. (optional)
ERROR 2
ACS values your privacy. By submitting your information, you are gaining access to C&EN and subscribing to our weekly newsletter. We use the information you provide to make your reading experience better, and we will never sell your data to third party members.
Synthesis
Synthetic Replicator Amplifies Itself
An autocatalytic molecule harnesses a dynamic combinatorial library to speed up its own creation
by Celia Henry Arnaud
November 24, 2008
| A version of this story appeared in
Volume 86, Issue 47
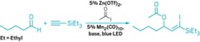
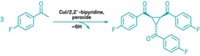
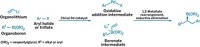
A molecule that can catalyze its own formation can also exploit reactions in a dynamic combinatorial library to amplify its formation at the expense of other species, Scottish researchers report (Angew. Chem. Int. Ed., DOI: 10.1002/anie.200804223). Douglas Philp and Jan W. Sadownik at the University of St. Andrews started with a pool of four reagents that react to form unreactive imines and reactive nitrones. The nitrones undergo irreversible dipolar cycloaddition reactions with a maleimide to form two pairs of diastereomeric cycloadducts, one of which can catalyze its own formation. This autocatalyzing replicator becomes the predominant product. Adding a small amount of the replicator as a template to the reagent pool causes it to become the predominant species even faster. "Chemical synthesis to date has focused on the creation of single chemical entities from carefully controlled reaction mixtures," Philp says. "We are trying to turn this around by having a general-purpose reagent pool that can be reconfigured as required. We see the application of this technology in nanoscale fabrication."



Join the conversation
Contact the reporter
Submit a Letter to the Editor for publication
Engage with us on Twitter