Advertisement
Grab your lab coat. Let's get started
Welcome!
Welcome!
Create an account below to get 6 C&EN articles per month, receive newsletters and more - all free.
It seems this is your first time logging in online. Please enter the following information to continue.
As an ACS member you automatically get access to this site. All we need is few more details to create your reading experience.
Not you? Sign in with a different account.
Not you? Sign in with a different account.
ERROR 1
ERROR 1
ERROR 2
ERROR 2
ERROR 2
ERROR 2
ERROR 2
Password and Confirm password must match.
If you have an ACS member number, please enter it here so we can link this account to your membership. (optional)
ERROR 2
ACS values your privacy. By submitting your information, you are gaining access to C&EN and subscribing to our weekly newsletter. We use the information you provide to make your reading experience better, and we will never sell your data to third party members.
Synthesis
Tamed Germanium
Cryptand cage sequesters a nearly naked germanium dication
by Bethany Halford
December 1, 2008
| A version of this story appeared in
Volume 86, Issue 48

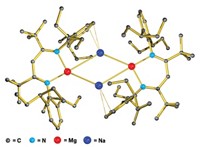
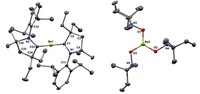
BY ENTOMBING a germanium dication within a cryptand molecule, chemists in Canada have managed to catch this highly reactive species. The work, published in Science (2008, 322, 1360), represents the first time that a doubly charged nonmetallic ion without any covalent bonds has been isolated. The discovery could provide an avenue for creating other so-called naked nonmetallic cations, such as Si2+ and P3+, the researchers say.
Isolating this novel reactive intermediate is "a remarkable and exciting achievement," notes Northwestern University chemistry professor Joseph B. Lambert in a commentary that accompanies the paper. "Under particular circumstances, reactive intermediates have been isolated and characterized, but establishing the existence of an entirely new class of intermediates is rare indeed," he adds.
After trying unsuccessfully to crystallize a crown ether complex of the elusive Ge2+, Paul A. Rupar, Kim M. Baines, and Viktor N. Staroverov of the University of Western Ontario turned to cryptand molecules. These bicyclic macromolecular polyether cages have been used to imprison metallic cations. Because a cryptand has the ability to completely surround an ion, the chemists reasoned that it could protect the remarkably reactive Ge2+ from nucleophilic counterions and solvent molecules.
Trapping the dication with the cryptand turned out to be surprisingly easy, Baines says. It worked on the first try, rapidly producing a white precipitate when the researchers mixed a germanium carbene complex with a cryptand. "It is just a beautiful reaction," Baines adds. "We hope it will inspire others to think of using cryptands in this area of chemistry."
Crystallographic studies indicate the Ge2+ sits halfway between the cryptand's nitrogen atoms and is equidistant from its oxygen atoms. Although the ion is too far away to form covalent bonds with any of these atoms, Lambert believes it is likely that each atom provides a "small amount of bonding." Consequently, he describes the germanium as "nearly naked," even though the germanium has been stripped of any bonding partners. "Without a doubt," he adds, it is "a species for which there is no precedent in nonmetallic inorganic chemistry."


Join the conversation
Contact the reporter
Submit a Letter to the Editor for publication
Engage with us on Twitter