Advertisement
Grab your lab coat. Let's get started
Welcome!
Welcome!
Create an account below to get 6 C&EN articles per month, receive newsletters and more - all free.
It seems this is your first time logging in online. Please enter the following information to continue.
As an ACS member you automatically get access to this site. All we need is few more details to create your reading experience.
Not you? Sign in with a different account.
Not you? Sign in with a different account.
ERROR 1
ERROR 1
ERROR 2
ERROR 2
ERROR 2
ERROR 2
ERROR 2
Password and Confirm password must match.
If you have an ACS member number, please enter it here so we can link this account to your membership. (optional)
ERROR 2
ACS values your privacy. By submitting your information, you are gaining access to C&EN and subscribing to our weekly newsletter. We use the information you provide to make your reading experience better, and we will never sell your data to third party members.
Business
The Power Of Pores
New polymer separators are gates to next-generation cars
by Alexander H. Tullo
February 18, 2008
| A version of this story appeared in
Volume 86, Issue 7

THE REALITY of driving to work using electric power may only be a hair away. That is about the thickness of the polymer separators used in lithium-ion batteries. Without them, there would be no such batteries or the portability they lend to everything from iPods to power tools. Chemical companies say these essential battery components are ready for the greater challenge of weaning automobiles off gasoline, and several of them are vying to lead the way.
If lithium-ion batteries were a movie, electrode materials would get top billing. Cathodes are based on lithium-transition-metal oxides, such as lithium cobalt oxide (LiCoO2), while a common anode material is lithium-intercalated carbon (LiC6). Dozens of other electrode chemistries have been developed to improve battery safety and energy density. But the principle behind all lithium batteries is the migration of lithium ions, via an electrolyte such as a lithium salt dissolved in an organic solvent, from cathode to anode on charging and in the other direction when discharging.
Separators are more like film directors in the behind-the-scenes role they play. They are electrically insulating porous polymer membranes usually composed of polyolefins in one or more layers. They prevent electrons from passing directly from anode to cathode, sending them off instead to power electronic devices. At the same time, separators let lithium ions pass between electrodes via their pores.
Pat Brant, ExxonMobil Chemical's chief polymer scientist, likens the separator to the human lung. "Getting the pores right is basically what allows the battery to breathe," he says. "The more porous the separator is, while still retaining good toughness characteristics, the more electrons you can pump out for the external circuit."
Without the separator, the anode and cathode would spontaneously react and the battery cell would fizzle. According to Kuzhikalail M. Abraham, a lithium battery consultant with E-KEM Sciences, a ruptured separator is a common cause of the catastrophic battery failures that have led to flaming laptops and the recall of millions of batteries. "To me, the separator is the key to the safety of the battery," he says.
Separators are thus crucial to automakers' plans to mass market electric and hybrid cars. Today, hybrids like the Toyota Prius use nickel-metal-hydride batteries. But automakers are looking to lithium-ion batteries for the next generation of electric vehicles for the same reasons electronics makers have taken to them. Lithium-ion batteries can store the same amount of energy as nickel-metal-hydride batteries in about half the mass, Brant says.
AUTOMAKERS ARE planning lithium-ion batteries for vehicles, such as plug-in hybrids, that could be charged from an electrical outlet. General Motors says it will install lithium batteries in the plug-in version of the Saturn Vue when it comes out in 2010.
But cars hold more challenges for batteries than consumer electronics. Safety is a huge consideration. Automakers can't risk even a rare battery fire given the sheer amounts of energy that will be stored.
Jon Lauckner, vice president of global program management for GM, told a forum sponsored by the National Chamber of Commerce last month in Washington, D.C., about additional challenges. "Our performance and durability requirements—10 years of life, 150,000 miles in a very rugged and hostile environment—are unique to automotive applications and considerably more stringent than those applied to consumer goods," he said.
According to Abraham, cars are very different from laptop computers, which draw a small amount of power over a long period of time. "For power tools and hybrid-electric vehicles, you need to run at a much higher rate," he says. "You design the electrode and the circuit to allow the electrochemical reaction to take place at a faster rate without building up heat."
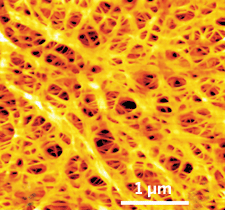
The pores in ExxonMobil's polymer separators allow batteries to "breathe."
ExxonMobil, which has supplied battery separator films for consumer electronics since the early 1990s, recently introduced a separator specifically targeted at the automotive market. One key to the new film is an increased porosity that allows greater ion flow and thus more power.
To produce the separators, ExxonMobil uses a wet process to dissolve polyethylene resin in an organic solvent. Evaporation of the solvent leaves behind a porous film. The company's breakthrough is to coextrude multiple film layers, each of which imparts different characteristics.
SOME OF THESE LAYERS promote safety. For instance, one layer shuts down the battery cell if it gets too hot. When the separator reaches 140 ??C, the membrane closes its pores, thereby stopping the flow of ions and preventing a runaway reaction. Because unintended reactions can still take place in the cell's complex chemical soup, the company has also boosted the thermal stability of the layers to withstand temperatures up to about 190 ??C. That prevents those reactions from rupturing the battery.
ExxonMobil separators are being used in the Maya-300, an electric car made by start-up Electrovaya. The car, intended for urban driving, has a range of 120 miles and a top speed of 30 mph. Brant says ExxonMobil's separators are also being tested in other electric vehicles.
Evonik Industries is marketing a separator for automotive and utility batteries. The material is a nonwoven polyethylene terephthalate film coated with a ceramic layer. Its electrical properties are similar to those of polyolefin-based separators, the company says, and advantages include longer life and enhanced safety features. E-KEM Sciences' Abraham says the material does have a higher melting point and greater mechanical strength than polyolefin separators.
Japan's Asahi Kasei, which claims to hold 50% of today's battery separator market, is also eyeing automotive applications. The company offers large- and small-pore versions of its polyolefin-based Hipore separators, made using a wet process, but will yield little technical information. "Separator properties really depend on cell design," says Satoru Yamaguchi, manager of the planning and coordination group of Asahi Kasei's Hipore and battery materials division. "All I can say here is that it is just like the tailor-made jacket," he says.
Celgard, a Charlotte, N.C.-based maker of separator films, is also participating in car battery programs, says Mitch Pulwer, vice president and general manager. The company uses a dry process that stretches a polyolefin to form a thin porous film.
According to Pulwer, Celgard has been modifying its multilayer films for greater porosity and melt strength to make them appropriate for automotive use. "We can make variations of current products," he says, "or develop new separators that might be needed."
Pulwer is excited by the opportunity that cars present. While attending the North American International Auto Show in Detroit last month, he noticed that every carmaker "in the world had a display on the auto show floor with power packs and lithium-ion batteries."
Brant agrees that interest is much higher than it was only two years ago. "There is now unanimity of opinion and purpose to the delivery of lithium batteries in mass production for vehicles," he says. "And our intention is to make that happen."


Join the conversation
Contact the reporter
Submit a Letter to the Editor for publication
Engage with us on Twitter