Advertisement
Grab your lab coat. Let's get started
Welcome!
Welcome!
Create an account below to get 6 C&EN articles per month, receive newsletters and more - all free.
It seems this is your first time logging in online. Please enter the following information to continue.
As an ACS member you automatically get access to this site. All we need is few more details to create your reading experience.
Not you? Sign in with a different account.
Not you? Sign in with a different account.
ERROR 1
ERROR 1
ERROR 2
ERROR 2
ERROR 2
ERROR 2
ERROR 2
Password and Confirm password must match.
If you have an ACS member number, please enter it here so we can link this account to your membership. (optional)
ERROR 2
ACS values your privacy. By submitting your information, you are gaining access to C&EN and subscribing to our weekly newsletter. We use the information you provide to make your reading experience better, and we will never sell your data to third party members.
Materials
Different Inside And Out
New route yields selectively functionalized porous materials
by Rachel Petkewich
March 3, 2008
| A version of this story appeared in
Volume 86, Issue 9
A new strategy for establishing distinct chemical functionalities on the inside and outside of mesoporous silicon could advance design of sensors and drug delivery systems (Angew. Chem. Int. Ed., DOI: 10.1002/anie.200704784). Such a route would be useful, for example, for making a material with a drug stored inside its pores and an external coating of antibody that targets the site where the drug is to be delivered.
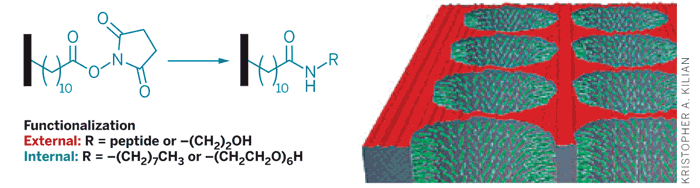
Researchers created a hydrophobic monolayer on the material's internal and external pore surfaces (black bars). Then both external (red) and internal (green) surfaces were selectively functionalized with two different moieties.
Mesoporous materials have pores that range in size from 2 to 50 nm, which is advantageous for applications that involve biomolecules. For the materials to perform some of their intended tasks, the internal and external surfaces of the pores must be modified with different groups. But current methods cannot reliably and distinctively functionalize internal and external surfaces.
Chemistry professor J. Justin Gooding at the University of New South Wales in Australia, and colleagues harnessed hydrophobicity to overcome these limitations. "We can couple completely different things to the inside and outside of a mesoporous material by using self-assembled monolayers on the surfaces," Gooding says. As a demonstration, the researchers immobilized ligands to promote cell adhesion on the exterior of a porous silicon crystal and then introduced different chemical groups on the internal pore walls.
The strategy required modifying both the internal and external surfaces of the pores to create a hydrophobic, succinimide ester-terminated monolayer via thermal hydrosilylation. Next, the researchers showed that the monolayer, aided by surface tension, prevents water from entering the pores. As a result, they could selectively derivatize the external surface with an aqueous solution of peptides. Finally, the researchers used an organic solvent to functionalize the inside of the pores with groups that resist proteins.
Gooding says their approach is not necessarily limited to silicon or mesoporous materials, but it would also apply to materials with pore sizes that are smaller (<2 nm) or larger (>50 nm).
Christopher C. Landry, a professor of chemistry at the University of Vermont, highlights the strategy's versatility for materials with various pore sizes but notes a limitation: At this point, the external surface must be modified by an aqueous solution, so groups that are water-insoluble cannot be added to the external surface.


Join the conversation
Contact the reporter
Submit a Letter to the Editor for publication
Engage with us on Twitter