Advertisement
Grab your lab coat. Let's get started
Welcome!
Welcome!
Create an account below to get 6 C&EN articles per month, receive newsletters and more - all free.
It seems this is your first time logging in online. Please enter the following information to continue.
As an ACS member you automatically get access to this site. All we need is few more details to create your reading experience.
Not you? Sign in with a different account.
Not you? Sign in with a different account.
ERROR 1
ERROR 1
ERROR 2
ERROR 2
ERROR 2
ERROR 2
ERROR 2
Password and Confirm password must match.
If you have an ACS member number, please enter it here so we can link this account to your membership. (optional)
ERROR 2
ACS values your privacy. By submitting your information, you are gaining access to C&EN and subscribing to our weekly newsletter. We use the information you provide to make your reading experience better, and we will never sell your data to third party members.
Synthesis
Boron Dreams
Priestley Medalist M. Frederick Hawthorne has some unfinished business
by Stephen K. Ritter
March 23, 2009
| A version of this story appeared in
Volume 87, Issue 12

BORON IS NOT BORING. Just a few minutes of conversation with M. Frederick Hawthorne, recipient of the 2009 Priestley Medal, will prove it.
"Boron is my element—not that I own boron, but it owns me," Hawthorne reflects after more than a half-century of carrying out boron research. "I think boron is one of the last easy frontiers for the widespread use of any element. After carbon, it is a second starting point for chemists to develop new products, especially pharmaceuticals and nanomaterials."
On Tuesday, the American Chemical Society will honor Hawthorne with the Priestley Medal, the society's highest honor, for his contributions to the advancement of inorganic chemistry, in particular for his pioneering boron studies. As Hawthorne is quick to point out, when he first started as a research chemist in the 1950s, little was known about boron's chemical properties. But he made an assumption that it should be possible to do anything with boron that could already be done with its next-door neighbor, carbon.
Boron can't be as all-encompassing as carbon, of course, Hawthorne admits. But in the periodic table, boron is the only element besides carbon that readily combines with itself to form analogs of aliphatic chains, aromatic ring systems, and organometallic systems, he says.
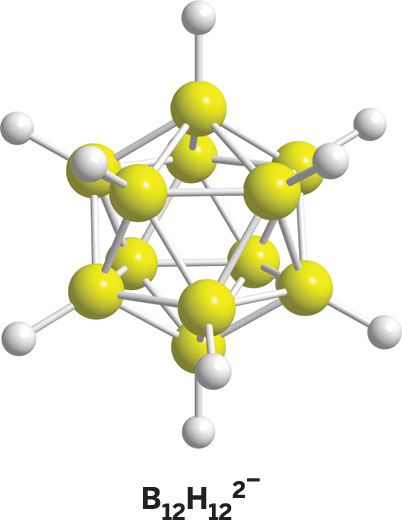
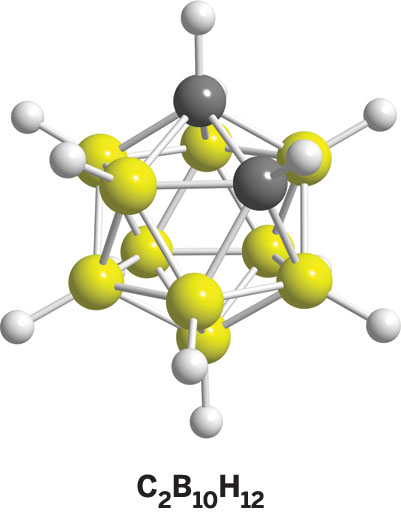
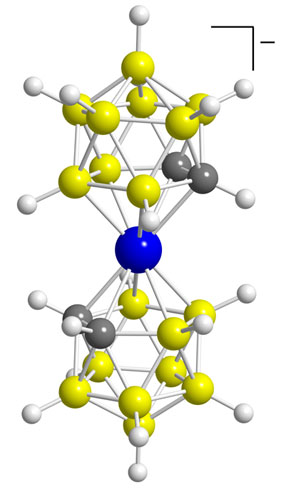
Hawthorne's initial gut feeling about boron has paid off. During his stints at Rohm and Haas; the University of California, Riverside; and UCLA, Hawthorne and his colleagues have created a diverse collection of polyhedral boranes, such as B12H122-, which structurally look like bird cages. His group has also produced a number of spin-off borane compounds, including the carboranes, such as C2B10H12, and the metallacarboranes, such as Ni(C2B9H11)2. Hawthorne has put these stable, low-toxicity compounds to work in applications as varied as catalysis, solid rocket fuels, medical imaging, neutron-based radiation treatments for cancers and rheumatoid arthritis, supramolecular host-guest complexes, and nanomachines.
And although Hawthorne is now 80, he is still far from giving up boron. To prove that, instead of retiring, three years ago he became founding director of the International Institute of Nano & Molecular Medicine at the University of Missouri, Columbia. A key goal of the institute, besides reinvigorating boron research, is to help fulfill Hawthorne's career-long dream of using his polyhedral boranes and carboranes for boron neutron capture therapy (BNCT) to cure common cancers.
"Hawthorne has expressed his creativity over and over again in chemistry," says supramolecular chemistry pioneer J. Fraser Stoddart, a friend and former UCLA colleague who is now at Northwestern University. "And let it not be forgotten that he coupled his stellar achievements in science for more than half of his career with his service as editor of Inorganic Chemistry, ACS's flagship inorganic journal."
When Stoddart thinks more deeply about Hawthorne, a pair of lines from Alfred Lord Tennyson's poem "The Brook" come to mind, he says. "For men may come and men may go, but I go on forever," Stoddart quotes. These lines speak to the enduring life of a stream as it passes through woods and farms, embellishing the existence of animate and inanimate objects that the waters touch. So it is with Hawthorne, Stoddart adds. "To many scientists the world over, he has become Mr. Inorganic Chemistry, a legend in his own lifetime."
Hawthorne's foray into boron chemistry came in 1956 during his first job as a research chemist at Rohm and Haas's lab at Redstone Arsenal army base in Huntsville, Ala. At that time, boron chemistry was mostly limited to the simple boron hydrides such as diborane (B2H6), pentaborane (B5H9), and decaborane (B10H14), Hawthorne explains. "In terms of organic chemistry, these compounds are alkyne, alkene, and alkane analogs," he notes.
Rohm and Haas chemists were working to develop borane-based solid propellants for rockets, Hawthorne relates. "We had NF compounds as oxidants and boranes as the reducing agents, and with that combination you could get super rockets."
With Hawthorne's "organic approach," he got boranes out of vacuum lines traditionally used to handle the compounds and into regular three-neck flasks on the bench in fume hoods. Within five years, Hawthorne's group came up with B10H102-, which was the first reported closed-cage polyhedral borane. Then came another key polyhedral borane, B12H122-. In terms of organic chemistry, these polyhedral dianions are analogs of aromatic hydrocarbons, such as benzene, Hawthorne says.
"Those discoveries turned out to really open up the field of borane chemistry," Hawthorne notes.
At about the same time, scientists in several research groups around the world reported the discovery of the first polyhedral carborane, C2B10H12. In the structure of this compound, which Hawthorne's group helped to elucidate, two carbon atoms have replaced two boron atoms of the B12H122- cage. Hawthorne's group showed that the polyhedral boranes and carboranes can be derivatized with many types of organic functional groups, resulting in myriad monomeric compounds, oligomers, and polymers.
In 1962, Hawthorne transitioned from industry to become a full professor of organic chemistry at the University of California's new campus in Riverside. It wasn't long before Hawthorne came up with another boron creation: metallacarboranes. Hawthorne already knew that hitting a carborane with a base would knock a boron atom out of one of the vertices of the polyhedral cage. The result is an open face, permitting the C2B9H112- carborane to function as a ligand, just like a cyclopentadienyl anion.
"We plugged up the opening with a metal to make the first metallacarborane, Fe(C2B9H11)2-, which created new opportunities for boron chemistry," Hawthorne says.
In 1968, chemists at UCLA expressed interest in Hawthorne's research and proposed that he help start an inorganic chemistry division there. He accepted the offer and moved his group. It was at UCLA that Hawthorne and colleagues began coming up with eclectic applications for their boranes and carboranes.
One avenue Hawthorne's group has explored is using metallacarboranes as homogeneous catalysts. Rhodium carborane complexes in particular have proven to be effective chiral catalysts for hydrogenation and isomerization reactions of alkenes and ketones under mild conditions.
Another application spawned by Hawthorne's group involves soluble and insoluble "carborods," which are rigid chains of carboranes with or without metal or organic spacers that are useful for making liquid crystals and molecular wires. In addition, "carboracycles" are molecular squares and rectangles formed from carboranes stitched together with linker units and designed for host-guest chemistry.
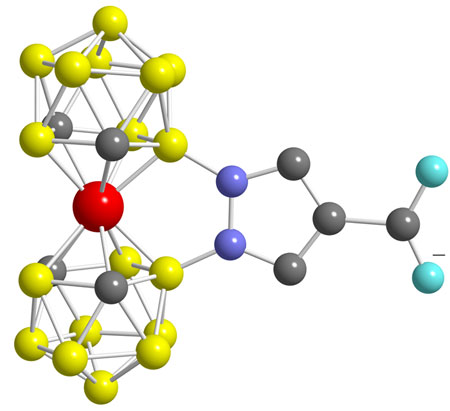
The metallacarboranes also are capable of hosting a positron-emitting isotope such as 55Co or the gamma-emitting 57Co for cancer imaging and treatment. Hawthorne's "Venus flytrap" complex, for instance, consists of a cobalt atom trapped between two carborane ligands. The complex includes a pyrazole ring that bridges the two carboranes to clamp them shut on the metal and provides a handle like a stem to attach a biomolecule such as a monoclonal antibody to target cancer tumors.
IN YET ANOTHER application, Hawthorne takes advantage of the oxidation-reduction properties of metallacarboranes to power moving parts of nanomachines. One machine built in Hawthorne's lab consists of a nickel atom nested between two C2B9H112- ligands. The ligands reversibly rotate 140º relative to each other as the metal "axle" is reduced and oxidized, motion that could be used to block and unblock pores or binding sites to control catalysts or enzymes, Hawthorne says.
"I have always been a huge fan of Hawthorne's work—his pioneering research discoveries constitute a cornerstone of modern chemistry," comments chemistry professor and nanoscience expert Chad A. Mirkin of Northwestern University.
"Hawthorne is someone who not only appreciates fundamental science and the importance of an interdisciplinary approach, but someone who is trying to transition that science into important new technologies," Mirkin adds. "I think his move to Missouri is an example of that idea, and I look forward to watching his continued success."
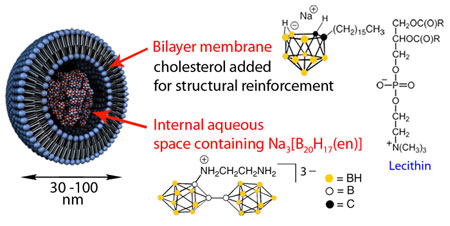
One of Hawthorne's most thrilling developments in his quest to develop BNCT is the creation of boron-laden liposomes. Liposomes are tiny cell-like bubbles with bilayer walls typically made from phospholipids, just like cell membranes. They are ideal vessels for selectively transporting anticancer drugs to tumors.

To create liposomes with a boron twist, Hawthorne's group uses amphiphilic carboranes, in which a hydrocarbon tail is appended to a charged carborane cage, as one component of the liposome bilayer wall. In addition to the high density of boron atoms in the liposome wall, water-soluble polyhedral borane dimers can be transported as cargo inside the liposomes.
Once the liposomes or other tumor-targeting boron compounds localize in tumor cells, the tumor is irradiated with a beam of low-energy neutrons, which pass harmlessly through most tissues. The 10B nuclei capture the neutrons, forming unstable 11B atoms that fission to produce 4He2+ (alpha particles) and 7Li3+ nuclei, along with gamma rays and excess kinetic energy, Hawthorne explains. The recoiling energetic particles travel a short distance through the cell, ionizing and critically damaging whatever they encounter, ultimately killing the cell. "It's like creating a nuclear explosion in a cancer cell," he says.
One limitation of BNCT is that the technique was initially monopolized by neurosurgeons and radiation oncologists who were almost solely interested in brain tumors, Hawthorne notes. All the funding, perhaps $1 billion at today's dollar value, was poured into one tumor, glioblastoma multiforme, he says.
"That's a very deadly brain tumor," Hawthorne observes. "It is relatively rare, luckily, but it's still incurable by any means." The inability of BNCT to be effective against glioblastoma disenchanted granting agencies and led the field of BNCT to a dead end around 2000, he says, although a few groups around the world are still working on new strategies.
"But I have always wanted to chase after other types of more common cancers, such as lung and prostate cancers," Hawthorne says. "These cancers are not always amenable to surgical procedures or chemotherapy, but are treatable by radiation."
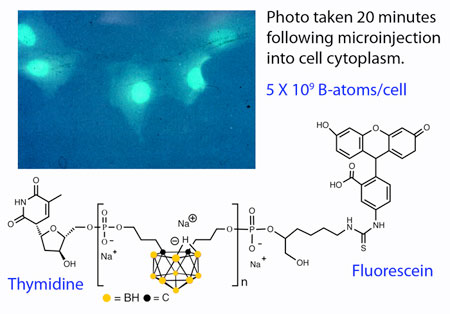
To that end, Hawthorne's group has used boron-containing liposomes to carry out numerous toxicity and biodistribution studies of boranes and carboranes in mice over the years, he says.
"We get tremendous uptake of the boron compounds in mice with high selectivity for tumors at low injected doses and low toxicity—all the good things you want," Hawthorne says. "But we never had a neutron source readily available where we could expose the mice, activate the boron, and try to save their bums." As a result, Hawthorne has never been able to prove that his liposome approach to BNCT works.
BUT AN INVITATION to give an after-dinner speech at the University of Missouri in spring 2005 changed all that. When Hawthorne got there, he met university officials and soon realized that the invitation was for more than just an after-dinner speech.
The University of Missouri has a medical school, veterinary school, engineering school, and the largest research nuclear reactor in the U.S., all on a single campus, Hawthorne points out. In one place there was everything he needed for BNCT, including eager scientists from every possible discipline willing to collaborate.
"That evening was a bit of a setup," Hawthorne now believes. "I was asked if I would be interested in relocating to head the new International Institute of Nano & Molecular Medicine." To make a long story short, he accepted.
The institute now has a new research building, which took only about 18 months to build—"a good example of a scientific barn raising," Hawthorne says. It is across the street from the research reactor, and a neutron beam line dedicated to exploratory BNCT research supports the two facilities.
"So that's where we are at the University of Missouri—a new beginning," Hawthorne says enthusiastically. Although the institute's scientists plan to investigate a wide variety of biomedical pursuits, the first order of business is BNCT. "Our plan is to start irradiating mice," he says. "Once we do mice, we will move to larger animals, and then finally to people. Perhaps within the next five years, human testing with our compounds may be possible."
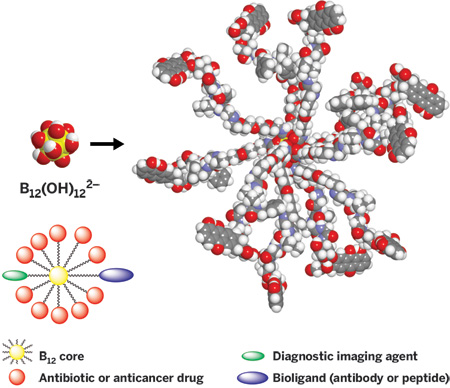
Hawthorne also aims to move beyond liposomes to develop nanoparticles using B12(OH)122- as a core. The B12(OH)122- anion acts much like a sugar, and the hydroxyl groups of these compounds are easily converted to esters, ethers, and carbamates for linking up payload molecules, he says.
Advertisement
The researchers have already shown that it's possible to functionalize the borane hydroxyl groups with an antibody or peptide for targeting to protein receptors in cells, fluorescent groups for optical monitoring, gadolinium complexes for magnetic resonance imaging, and antibiotics or anticancer drugs that can work in concert with BNCT. The 12 boron sites allow delivery of many copies of a single reagent or, in the ultimate case, a combination of functional groups that can target specific tissues, facilitate imaging, and carry out life-saving therapy all in one blow, Hawthorne notes.
"It has been a long road," Hawthorne says, but he thinks he is finally on the verge of seeing his life's work come to full fruition. He believes boranes and carboranes will eventually be as ubiquitous in medical diagnostics and pharmaceuticals as organic compounds. Hawthorne isn't waiting around hoping that will happen; in his typical dynamic fashion, he is seeing to it that it will happen.





Join the conversation
Contact the reporter
Submit a Letter to the Editor for publication
Engage with us on Twitter