Advertisement
Grab your lab coat. Let's get started
Welcome!
Welcome!
Create an account below to get 6 C&EN articles per month, receive newsletters and more - all free.
It seems this is your first time logging in online. Please enter the following information to continue.
As an ACS member you automatically get access to this site. All we need is few more details to create your reading experience.
Not you? Sign in with a different account.
Not you? Sign in with a different account.
ERROR 1
ERROR 1
ERROR 2
ERROR 2
ERROR 2
ERROR 2
ERROR 2
Password and Confirm password must match.
If you have an ACS member number, please enter it here so we can link this account to your membership. (optional)
ERROR 2
ACS values your privacy. By submitting your information, you are gaining access to C&EN and subscribing to our weekly newsletter. We use the information you provide to make your reading experience better, and we will never sell your data to third party members.
Materials
Photonic Polyethylene
Unique block copolymers yield colorful, low-cost materials for many applications
by Stephen K. Ritter
May 4, 2009
| A version of this story appeared in
Volume 87, Issue 18

DOW CHEMICAL scientists have used their unique "chain-shuttling" polymerization technique to make polyethylene block copolymers that can self-assemble into materials with ordered phases that give rise to colorful optical properties (Macromolecules, DOI: 10.1021/ma9002819). Compared with colloidal crystals and other photonic materials, these photonic polyethylenes could become a low-cost staple polymer for a new range of applications, including energy-efficient building materials that reflect sunlight.
In 2006, a Dow Chemical team identified a pair of catalysts that work in combination to build polyethylene block copolymers from ethylene and 1-octene (C&EN, May 8, 2006, page 9). The process takes advantage of diethylzinc as a transfer reagent to shuttle the growing polymer chains back and forth between the catalysts in a continuous reaction that adds alternating blocks of ethylene and 1-octene with precise control over polymer composition.
Now, Phillip D. Hustad, Jeffrey D. Weinhold, Gary R. Marchand, Eddy I. Garcia-Meitin, and Patricia L. Roberts at the company's Freeport, Texas, site have modified the technique to make polydisperse polyethylene diblock copolymers with a distribution of block lengths. When melted and compressed into films, the distinct polymeric segments self-assemble into a layered pattern of "hard" semicrystalline and "soft" amorphous phases, Hustad explains. Each phase has a different refractive index, which enables the polymer films to function as photonic crystals and scatter visible light.
Block copolymers have been used to form photonic crystals in the past, Hustad says, but achieving structural features large enough to interact with visible light has proven difficult. Such materials have only been prepared by using very high molecular weight copolymers, typically up to 1 million g/mol, and maintaining uniform (monodisperse) polymer chain lengths, which requires solution processing that adds complexity and cost.
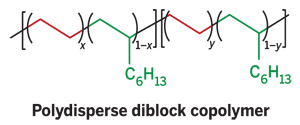
The Dow Chemical polydisperse polyolefin diblock copolymers achieve the same effects with an order of magnitude lower molecular weight and without solvent processing. In addition, the semicrystalline nature of the new materials allows them to be reversibly switched from reflective to nonreflective states by heating and cooling.
Polymer chemist Marc A. Hillmyer of the University of Minnesota, Twin Cities, says the Dow Chemical approach allows the synthesis of polymers that "simply cannot be prepared by conventional methods." The research is "a nice example of how increased polydispersity can actually be beneficial and lead to new phenomena in certain block copolymer systems," Hillmyer notes.
MIT physicist Edwin L. Thomas, an expert in photonic polymers, adds that the Dow Chemical approach "presents a real opportunity to implement photonic polymers for many low-cost applications."



Join the conversation
Contact the reporter
Submit a Letter to the Editor for publication
Engage with us on Twitter