Advertisement
Grab your lab coat. Let's get started
Welcome!
Welcome!
Create an account below to get 6 C&EN articles per month, receive newsletters and more - all free.
It seems this is your first time logging in online. Please enter the following information to continue.
As an ACS member you automatically get access to this site. All we need is few more details to create your reading experience.
Not you? Sign in with a different account.
Not you? Sign in with a different account.
ERROR 1
ERROR 1
ERROR 2
ERROR 2
ERROR 2
ERROR 2
ERROR 2
Password and Confirm password must match.
If you have an ACS member number, please enter it here so we can link this account to your membership. (optional)
ERROR 2
ACS values your privacy. By submitting your information, you are gaining access to C&EN and subscribing to our weekly newsletter. We use the information you provide to make your reading experience better, and we will never sell your data to third party members.
Materials
Platinum Catalysts Branch Out
Dendritic structures outperform current fuel-cell materials
by Bethany Halford
May 18, 2009
| A version of this story appeared in
Volume 87, Issue 20

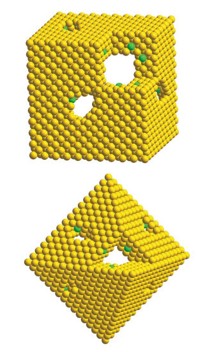
BY CAREFULLY CULTIVATING treelike nanostructures, scientists have created fuel-cell catalysts that are more than twice as active as state-of-the-art materials with the same mass (Science, DOI: 10.1126/science.1170377). The so-called bimetallic nanodendrites, which feature a palladium core surrounded by densely packed platinum branches, could lower the cost of fuel-cell-cathode materials, making the technology more viable for automotive applications.
The nanodendrites make superior catalysts because their branched structure creates a large surface area and maximizes the exposure of the Pt facets that are most active in the oxygen-reducing reaction that takes place in a fuel cell's cathode, according to Younan Xia, the engineering professor at Washington University in St. Louis who spearheaded the research. The treelike nanostructures are two-and-a-half times more active than the fine particles of Pt supported on porous carbon currently used in fuel-cell cathodes.
Xia's team creates the nanodendrites by reducing K2PtCl4 with L-ascorbic acid in the presence of faceted Pd nanocrystal seeds and a stabilizing polymer. "This synthetic protocol could be readily scaled up to become practical," Xia tells C&EN.
He also notes that the process is environmentally benign because it takes place in water without the need for high temperatures or electrochemical deposition. Xia and colleagues are working to improve the durability of the nanodendrites, which are currently only slightly more durable than commercial Pt-based catalysts.
The bimetallic nanodendrite catalysts represent "exciting progress in developing a new approach for effective usage of Pt for energy science," comments Zhong Lin Wang, a materials science professor at Georgia Tech. "The demand for Pt is growing each day. Therefore, searching for new catalysts to replace Pt or improving the effectiveness of Pt for catalysis is of vital importance for energy science and chemical technology," he says.




Join the conversation
Contact the reporter
Submit a Letter to the Editor for publication
Engage with us on Twitter