Advertisement
Grab your lab coat. Let's get started
Welcome!
Welcome!
Create an account below to get 6 C&EN articles per month, receive newsletters and more - all free.
It seems this is your first time logging in online. Please enter the following information to continue.
As an ACS member you automatically get access to this site. All we need is few more details to create your reading experience.
Not you? Sign in with a different account.
Not you? Sign in with a different account.
ERROR 1
ERROR 1
ERROR 2
ERROR 2
ERROR 2
ERROR 2
ERROR 2
Password and Confirm password must match.
If you have an ACS member number, please enter it here so we can link this account to your membership. (optional)
ERROR 2
ACS values your privacy. By submitting your information, you are gaining access to C&EN and subscribing to our weekly newsletter. We use the information you provide to make your reading experience better, and we will never sell your data to third party members.
Synthesis
Surprise Methyl C–H Activation In DMF
Caltech researchers find that a methyl C–H bond in dimethylformamide can be preferentially activated over the labile aldehyde C–H bond
by Stephen K. Ritter
June 15, 2009
| A version of this story appeared in
Volume 87, Issue 24
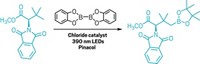
In a synthetic first, California Institute of Technology's Valerie J. Scott, John E. Bercaw, Jay A. Labinger, and coworkers have found that one of the methyl C–H bonds in dimethylformamide (DMF) can be preferentially activated by iridium over the labile aldehyde C–H bond (Organometallics, DOI: 10.1021/om9002413). DMF, (CH3)2NCHO, is a common solvent and widely used as a reagent for introducing carbonyl groups into inorganic and organic molecules. Even so, the mechanism of DMF-based carbonylation reactions is not well-known. The expected carbonylation pathway begins with activation of the aldehyde C–H bond. But when the Caltech team set out to synthesize iridium carbonyl iodide complexes for one of its research projects, the researchers serendipitously found a side reaction in which C–H activation occurred at the methyl end of the molecule, leaving the aldehyde group intact. They observed the C–H switch in the unusual iridium complex shown, which was isolated as a minor product after refluxing IrCl3 with DMF under acidic conditions. "The fact that methyl C–H activation can compete at all with activation of the much more reactive aldehydic C–H bond is unexpected and intriguing," the researchers note.




Join the conversation
Contact the reporter
Submit a Letter to the Editor for publication
Engage with us on Twitter