Advertisement
Grab your lab coat. Let's get started
Welcome!
Welcome!
Create an account below to get 6 C&EN articles per month, receive newsletters and more - all free.
It seems this is your first time logging in online. Please enter the following information to continue.
As an ACS member you automatically get access to this site. All we need is few more details to create your reading experience.
Not you? Sign in with a different account.
Not you? Sign in with a different account.
ERROR 1
ERROR 1
ERROR 2
ERROR 2
ERROR 2
ERROR 2
ERROR 2
Password and Confirm password must match.
If you have an ACS member number, please enter it here so we can link this account to your membership. (optional)
ERROR 2
ACS values your privacy. By submitting your information, you are gaining access to C&EN and subscribing to our weekly newsletter. We use the information you provide to make your reading experience better, and we will never sell your data to third party members.
Physical Chemistry
Unusual Aluminum Bonding Geometry
Catalysis: Pentacoordinated Al joins Pt in supported catalysts
by Mitch Jacoby
September 28, 2009
| A version of this story appeared in
Volume 87, Issue 39

Aluminum atoms with fivefold bonding coordination serve as atomic posts that anchor platinum in alumina-supported platinum catalysts, chemists at Pacific Northwest National Laboratory (PNNL) report in Science (2009, 325, 1670). The study provides a molecular-scale view of bonding interactions in one of the most common commercial catalysts and points to strategies for improving it.
Precious metals dispersed on solid oxides constitute a large fraction of industrial catalysts. Alumina-supported platinum, for example, facilitates a variety of hydrocarbon-conversion processes, such as the ones that purify engine emissions in automobile catalytic converters. To derive high activity from such catalysts, the metal must be dispersed finely because platinum atoms buried in the interior of catalyst particles cannot drive chemical reactions.
The extent of metal dispersion is related to the strength and nature of the interaction between the metal atoms and oxide support. Yet molecular-level details of the bonds that attach the metal to the support are difficult to discern and have been generally unknown.
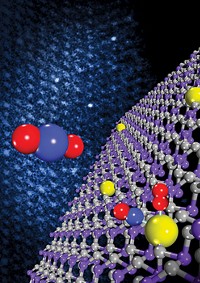
PNNL chemists Ja Hun Kwak, Charles H. F. Peden, and Janos Szanyi and their coworkers have now determined that platinum atoms use oxygen bridges to bind to pentacoordinated Al3+ sites on γ-Al2O3, a common form of alumina. Based on high-field nuclear magnetic resonance spectroscopy, electron microscopy, and computational analysis, the study shows that platinum assembles into thin, two-dimensional "rafts" that "float" above the support at the point on which the raft is anchored.
The study also shows that the energetics of changing aluminum's coordination number from five to the more usual six drives formation of the 2-D structures. Importantly, the number of "penta" sites can be increased by raising the temperature during catalyst synthesis, the team notes.
Catalyst specialist Bert M. Weckhuysen of Utrecht University, in the Netherlands, finds this study to be "beautiful and highly relevant" because it addresses an important and widely used catalytic material and suggests new ways to improve it.


Join the conversation
Contact the reporter
Submit a Letter to the Editor for publication
Engage with us on Twitter