Advertisement
Grab your lab coat. Let's get started
Welcome!
Welcome!
Create an account below to get 6 C&EN articles per month, receive newsletters and more - all free.
It seems this is your first time logging in online. Please enter the following information to continue.
As an ACS member you automatically get access to this site. All we need is few more details to create your reading experience.
Not you? Sign in with a different account.
Not you? Sign in with a different account.
ERROR 1
ERROR 1
ERROR 2
ERROR 2
ERROR 2
ERROR 2
ERROR 2
Password and Confirm password must match.
If you have an ACS member number, please enter it here so we can link this account to your membership. (optional)
ERROR 2
ACS values your privacy. By submitting your information, you are gaining access to C&EN and subscribing to our weekly newsletter. We use the information you provide to make your reading experience better, and we will never sell your data to third party members.
Materials
A New Blue
Inorganic Chemistry: Mn-based chromophore points to more planet-friendly pigments
by Bethany Halford
November 16, 2009
| A version of this story appeared in
Volume 87, Issue 46

By swapping a few indium atoms with manganese, chemists have created a new blue chromophore (J. Am. Chem. Soc., DOI: 10.1021/ja9080666). The Mn-doped substance suggests a route to compounds that could replace existing blue pigments with ones that are cheaper, more stable, and environmentally benign, the researchers say.
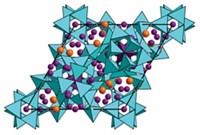
“We were not looking for a blue pigment,” confesses Oregon State University professor Mas A. Subramanian, who led the research effort. “In fact, we were actually looking for a multiferroic material”—both ferromagnetic and ferroelectric, for instance—and toward that goal were doping YInO3 with Mn to make YIn1- xMnxO3. Although Subramanian’s group expected to pull black or gray material from their furnace, they were surprised to see a bright blue powder instead. “I had never seen anything like this in all my years working with metal oxides,” he recalls.
Because YInO3 is white and YMnO3 is black, Subramanian wondered what made the new compound blue. In collaboration with University of California, Santa Barbara’s Nicola A. Spaldin, his group determined that the color comes from the unusual trigonal bipyramidal coordination of Mn3+. This gives rise to energy levels in manganese’s d orbitals that result in an intense absorption in the red/green region of the visible spectrum.
The reported new composition is unlikely to be used for large-scale pigmentation because of indium’s cost and toxicity, Subramanian notes. But his team found they were able to create other blue chromophores by doping Mn3+ into trigonal bipyramidal sites in other metal oxides, such as LuGaMgO4. They are using this strategy to design cost-effective blue pigments using cheaper materials.
“Today we are still using historical blue pigments like cobalt blue and Prussian blue, despite the fact that there are issues with their toxicity,” says Patrick M. Woodward, a solid-state chemistry expert at Ohio State University. “Subramanian and coworkers have found a simple route to a family of environmentally benign, chemically and thermally stable, bright blue inorganic pigments,” he says.





Join the conversation
Contact the reporter
Submit a Letter to the Editor for publication
Engage with us on Twitter