Advertisement
Grab your lab coat. Let's get started
Welcome!
Welcome!
Create an account below to get 6 C&EN articles per month, receive newsletters and more - all free.
It seems this is your first time logging in online. Please enter the following information to continue.
As an ACS member you automatically get access to this site. All we need is few more details to create your reading experience.
Not you? Sign in with a different account.
Not you? Sign in with a different account.
ERROR 1
ERROR 1
ERROR 2
ERROR 2
ERROR 2
ERROR 2
ERROR 2
Password and Confirm password must match.
If you have an ACS member number, please enter it here so we can link this account to your membership. (optional)
ERROR 2
ACS values your privacy. By submitting your information, you are gaining access to C&EN and subscribing to our weekly newsletter. We use the information you provide to make your reading experience better, and we will never sell your data to third party members.
Physical Chemistry
Modulating Electron Transfer
Using an infrared beam to control the flow of electrons across a H-bond bridge could lead to a new type of molecular switch
by Elizabeth K. Wilson
December 7, 2009
| A version of this story appeared in
Volume 87, Issue 49

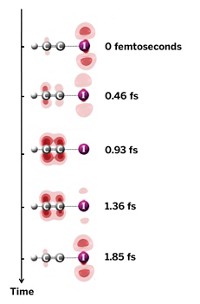
Tulane University’s Igor V. Rubtsov and Duke University’s David N. Beratan report that they can control the flow of electrons across a molecular bridge by exciting the bridge with infrared radiation. The discovery, which the authors say is a first, lends itself to the possibility of designing IR-controlled molecular switches and other devices (J. Am. Chem. Soc., DOI: 10.1021/ja907041t). The researchers designed a molecular ensemble containing a dimethylaniline-based electron donor on one end and an anthracene-derived electron acceptor on the other end. The gap in between was filled by hydrogen-bonded guanosine and cytidine nucleobases. The group, which included Spiros S. Skourtis of the University of Cyprus and Jonathan L. Sessler of the University of Texas, Austin, studied the rate of electron transfer across the bridge, first with a pulse of UV light that set off the electron-transfer process, followed by an IR pulse directed at the bridge’s vibrational modes. The IR excitation slowed the rate of electron transfer, they note, likely because the molecular vibrations either disrupted the bridging H-bonds or distorted the π-electron system of the bridge.


Join the conversation
Contact the reporter
Submit a Letter to the Editor for publication
Engage with us on Twitter