Advertisement
Grab your lab coat. Let's get started
Welcome!
Welcome!
Create an account below to get 6 C&EN articles per month, receive newsletters and more - all free.
It seems this is your first time logging in online. Please enter the following information to continue.
As an ACS member you automatically get access to this site. All we need is few more details to create your reading experience.
Not you? Sign in with a different account.
Not you? Sign in with a different account.
ERROR 1
ERROR 1
ERROR 2
ERROR 2
ERROR 2
ERROR 2
ERROR 2
Password and Confirm password must match.
If you have an ACS member number, please enter it here so we can link this account to your membership. (optional)
ERROR 2
ACS values your privacy. By submitting your information, you are gaining access to C&EN and subscribing to our weekly newsletter. We use the information you provide to make your reading experience better, and we will never sell your data to third party members.
Materials
New Form Of Boron
Entity has significant ionic character, a first for a material made from a single element
by Carmen Drahl
February 2, 2009
| A version of this story appeared in
Volume 87, Issue 5
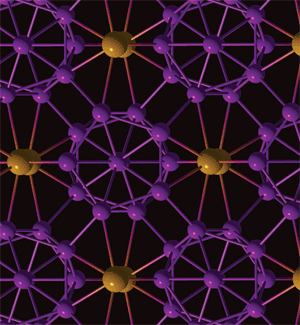
High-pressure tactics have uncovered a never-before-seen form of the element boron. The new entity has significant ionic character, a first for a material made from a single element.
"Boron seems to break all the rules and stereotypes of chemical bonding," says Artem R. Oganov, a theoretical crystallographer at the State University of New York, Stony Brook. Wedged between metals and nonmetals on the periodic table, boron adopts a range of structures, all of which are sensitive to impurities. As a result, researchers don't have a complete picture of boron's elemental forms.
Oganov led a multi-institution team that synthesized the new entity (Nature, DOI: 10.1038/nature07736). Although it takes shape only at elevated pressure, this new form remains stable under a wide range of temperatures and pressures.
The structure of the all-boron lattice is shown to the left. The team determined through computer simulations that it comprises clusters of 12 boron atoms interspersed with pairs of boron atoms. Each piece of the lattice transfers charges to the other, with the B12 units (purple) carrying a partial negative charge and the B2 (orange) units a partial positive charge.
"The work not only enriches our understanding of boron chemistry, it also strengthens parallels with gallium chemistry—the new structure's B2 units are reminiscent of Ga2 units found in gallium," comments François P. Gabbaï, a boron chemistry expert at Texas A&M University.


Join the conversation
Contact the reporter
Submit a Letter to the Editor for publication
Engage with us on Twitter