Advertisement
Grab your lab coat. Let's get started
Welcome!
Welcome!
Create an account below to get 6 C&EN articles per month, receive newsletters and more - all free.
It seems this is your first time logging in online. Please enter the following information to continue.
As an ACS member you automatically get access to this site. All we need is few more details to create your reading experience.
Not you? Sign in with a different account.
Not you? Sign in with a different account.
ERROR 1
ERROR 1
ERROR 2
ERROR 2
ERROR 2
ERROR 2
ERROR 2
Password and Confirm password must match.
If you have an ACS member number, please enter it here so we can link this account to your membership. (optional)
ERROR 2
ACS values your privacy. By submitting your information, you are gaining access to C&EN and subscribing to our weekly newsletter. We use the information you provide to make your reading experience better, and we will never sell your data to third party members.
Synthesis
Catalysis In Crystals
Inorganic Chemistry: Single crystals of iridium complex can exchange ligands and hydrogenate ethylene
by Jyllian N. Kemsley
June 7, 2010
| A version of this story appeared in
Volume 88, Issue 23
A single crystal of an iridium complex can exchange small-molecule ligands, such as CO and NH3, for N2 and selectively catalyze the conversion of ethylene to ethane, report researchers from the University of North Carolina, Chapel Hill (Nature 2010, 465, 598).
“Reactions of molecular crystals with gases involving covalent bond breaking and formation are as yet quite a rare observation,” says Lee Brammer, a chemistry professor at the University of Sheffield, in England. That the nonporous molecular crystals studied by the UNC group can hydrogenate ethylene is groundbreaking, he adds.
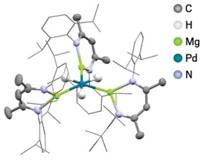
In the new work, Zheng Huang, Peter S. White, and Maurice Brookhart used a bulky, electron-deficient ligand, C6H3[OP(C6H2(CF3)3)2]2, and N2 to create the square planar iridium complex [Ir]–N2 (shown). Crystallization of the complex from toluene yielded light red single crystals. Structures of the crystals showed that the iridium complex’s four CF3-substituted aryl rings form a pocket around the N2 site. Multiple iridium complexes stack in such a way that disordered toluene molecules line a channel that runs along the small-molecule site.
When they exposed the [Ir]–N2 crystals to gases of small molecules, Huang and colleagues found that the N2 exchanged to form crystals of different colors: dark red [Ir]–NH3, orange [Ir]–CO (end-on with an Ir–C bond), deep red [Ir]–C2H4 (side-on), green [Ir]–O2 (side-on), and light red [Ir]–(H)2(H2) (two Ir–H bonds and one side-on H2). The crystals’ ability to exchange ligands depends on the size of the incoming molecule. For example, although the crystals took up ethylene to form [Ir]–C2H4, they would not take up propylene.
Huang and coworkers believe that the gases enter the crystal through the channel occupied by the disordered toluenes. Vacuum experiments demonstrated that the small-molecule ligands are not simply lost, leaving an open coordination site for the new ligand to fill. Instead, a ligand-exchange mechanism seems to be at work.
The group also found that when crystals of [Ir]–N2 were exposed to a mix of ethylene, propylene, and hydrogen, ethylene was selectively hydrogenated. That selectivity is not seen in common heterogeneous palladium-on-carbon hydrogenation catalysts, Sheffield’s Brammer notes.
Although growing single crystals would be impractical for large-scale catalysis, details gained from studying this system could provide insight into the catalytic mechanism and lead to better catalyst design, says Paul R. Raithby, a chemistry professor at the University of Bath, in England. Raithby points to measurements of solid-gas reaction kinetics and the possible development of new gas sensors based on single crystals as possible applications of the work.



Join the conversation
Contact the reporter
Submit a Letter to the Editor for publication
Engage with us on Twitter