Advertisement
Grab your lab coat. Let's get started
Welcome!
Welcome!
Create an account below to get 6 C&EN articles per month, receive newsletters and more - all free.
It seems this is your first time logging in online. Please enter the following information to continue.
As an ACS member you automatically get access to this site. All we need is few more details to create your reading experience.
Not you? Sign in with a different account.
Not you? Sign in with a different account.
ERROR 1
ERROR 1
ERROR 2
ERROR 2
ERROR 2
ERROR 2
ERROR 2
Password and Confirm password must match.
If you have an ACS member number, please enter it here so we can link this account to your membership. (optional)
ERROR 2
ACS values your privacy. By submitting your information, you are gaining access to C&EN and subscribing to our weekly newsletter. We use the information you provide to make your reading experience better, and we will never sell your data to third party members.
Synthesis
Cagey Arene Cleavage
Bis-carborane and ruthenium team up to facilitate difficult room-temperature aromatic C–C bond scission
by Carmen Drahl
June 21, 2010
| A version of this story appeared in
Volume 88, Issue 25

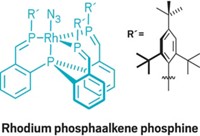
A carbon-boron cage compound has been discovered doing some challenging chemistry at room temperature—breaking the carbon-carbon bond of an aromatic ring. If a catalytic version of this chemistry could be developed, it might be useful for removing impurities in crude petroleum, the reaction’s discoverers say (Angew. Chem. Int. Ed., DOI: 10.1002/anie.201001555). Chemists have previously cleaved a C–C bond on an aromatic heterocycle at elevated temperatures (C&EN, Feb. 1, page 10). For Stuart A. Macgregor and Alan J. Welch of Scotland’s Heriot-Watt University, their room-temperature arene bond-breaking came unexpectedly. With a ruthenium p-cymene species, their team was trying to add a ruthenium vertex to each of the cages on a bis-carborane. But instead, one cage incorporated two ruthenium p-cymene fragments, and an aromatic C–C bond on one p-cymene was cleaved. Density functional theory calculations carried out by the team suggest that this happened because the p-cymene becomes sandwiched between two ruthenium atoms atop one carborane cage. The other carborane reduces the arene, disrupting its aromaticity and triggering bond-breaking. Adaptations of this chemistry to other arenes are in progress, Welch says.


Join the conversation
Contact the reporter
Submit a Letter to the Editor for publication
Engage with us on Twitter