Advertisement
Grab your lab coat. Let's get started
Welcome!
Welcome!
Create an account below to get 6 C&EN articles per month, receive newsletters and more - all free.
It seems this is your first time logging in online. Please enter the following information to continue.
As an ACS member you automatically get access to this site. All we need is few more details to create your reading experience.
Not you? Sign in with a different account.
Not you? Sign in with a different account.
ERROR 1
ERROR 1
ERROR 2
ERROR 2
ERROR 2
ERROR 2
ERROR 2
Password and Confirm password must match.
If you have an ACS member number, please enter it here so we can link this account to your membership. (optional)
ERROR 2
ACS values your privacy. By submitting your information, you are gaining access to C&EN and subscribing to our weekly newsletter. We use the information you provide to make your reading experience better, and we will never sell your data to third party members.
Synthesis
Mechanism Of Suzuki-Miyaura Coupling Revealed
Surprising findings could help researchers optimize coupling reactions
by Stu Borman
June 28, 2010
| A version of this story appeared in
Volume 88, Issue 26
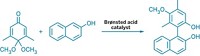
Trifluoroborate reagents are widely used as higher-yielding alternatives to traditional boronic acids in Suzuki-Miyaura coupling reactions, the palladium-catalyzed union of organoboron compounds with organohalides. But the mechanism of the trifluoroborate-based reactions (such as the one shown) has not been well understood. Now, Guy C. Lloyd-Jones of the University of Bristol, in England, and coworkers report using NMR, kinetics, isotopic labeling, and computation to investigate the mechanism (Angew. Chem. Int. Ed., DOI: 10.1002/anie.201001522). The study comes to a surprising conclusion—that trifluoroborates are hydrolyzed in the reactions to liberate the same boronic acids they were supposed to have replaced. Lloyd-Jones and colleagues show how several key mechanistic processes that result from trifluoroborate hydrolysis account for the reactions’ high efficiency, and they point out that these principles can now be used to optimize these and other reactions. For example, they note that boronic acid can couple with equally high efficiency as trifluoroborate if it is introduced into the reaction mixture in a slow and deliberate manner.



Join the conversation
Contact the reporter
Submit a Letter to the Editor for publication
Engage with us on Twitter