Advertisement
Grab your lab coat. Let's get started
Welcome!
Welcome!
Create an account below to get 6 C&EN articles per month, receive newsletters and more - all free.
It seems this is your first time logging in online. Please enter the following information to continue.
As an ACS member you automatically get access to this site. All we need is few more details to create your reading experience.
Not you? Sign in with a different account.
Not you? Sign in with a different account.
ERROR 1
ERROR 1
ERROR 2
ERROR 2
ERROR 2
ERROR 2
ERROR 2
Password and Confirm password must match.
If you have an ACS member number, please enter it here so we can link this account to your membership. (optional)
ERROR 2
ACS values your privacy. By submitting your information, you are gaining access to C&EN and subscribing to our weekly newsletter. We use the information you provide to make your reading experience better, and we will never sell your data to third party members.
Pharmaceuticals
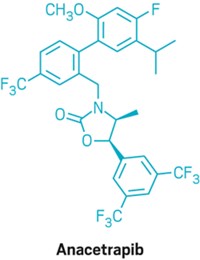
Merck Advances Cholesterol Drugs
Clinical Trials: Positive new data for both Vytorin and anacetrapib
by Lisa M. Jarvis
November 29, 2010
| A version of this story appeared in
Volume 88, Issue 48
Merck & Co. has unveiled promising results from clinical trials of two cholesterol-lowering drugs—one on the market, the other in development—that could be important to the company’s long-term health.
In a study of more than 9,000 people with chronic kidney disease, Vytorin lowered the incidence of heart attacks, strokes, and revascularization surgeries by 16%. According to Merck, no other drug has that effect for such patients.
The results come two years after the value of Vytorin, a combination of ezetimibe and the generic drug simvastatin, was called into question. A large clinical trial showed it was no better than simvastatin alone at preventing the buildup of arterial plaque.
Despite the good news, analysts haven’t changed their outlook for Vytorin. Pfizer’s Lipitor loses patent protection next year, and every cholesterol-lowering drug is expected to be hit by the influx of competition from generics. Deutsche Bank forecasts that sales for the Vytorin franchise will fall from $4.1 billion this year to $3.4 billion by 2014.
At the same time, Lipitor’s patent expiry puts the spotlight on new cholesterol drugs. Merck also came out with highly anticipated results from a Phase III trial of anacetrapib, a member of a family of compounds that raise the levels of “good” cholesterol by blocking cholesteryl ester transfer protein (CETP). In a trial of 1,623 people at high risk for heart disease, adding anacetrapib to their statin therapy resulted in a 138% increase in good cholesterol and a 40% decrease in “bad” cholesterol.
Anacetrapib has been closely watched after the colossal failure of torcetrapib, Pfizer’s CETP inhibitor. In 2006, Pfizer ended development of the compound because of a higher mortality rate among patients taking torcetrapib and Lipitor than those taking Lipitor alone. The deaths were later linked to biomarkers that Merck claims anacetrapib doesn’t affect.
Although the drug is still several years from the market, analysts are excited about the study results. “This clearly lays the groundwork for anacetrapib, and possibly other CETP inhibitors, to potentially be very important medicines in the mega-category of lipid modifiers,” Bernstein Research analyst Tim Anderson wrote in a note to investors.




Join the conversation
Contact the reporter
Submit a Letter to the Editor for publication
Engage with us on Twitter