Advertisement
Grab your lab coat. Let's get started
Welcome!
Welcome!
Create an account below to get 6 C&EN articles per month, receive newsletters and more - all free.
It seems this is your first time logging in online. Please enter the following information to continue.
As an ACS member you automatically get access to this site. All we need is few more details to create your reading experience.
Not you? Sign in with a different account.
Not you? Sign in with a different account.
ERROR 1
ERROR 1
ERROR 2
ERROR 2
ERROR 2
ERROR 2
ERROR 2
Password and Confirm password must match.
If you have an ACS member number, please enter it here so we can link this account to your membership. (optional)
ERROR 2
ACS values your privacy. By submitting your information, you are gaining access to C&EN and subscribing to our weekly newsletter. We use the information you provide to make your reading experience better, and we will never sell your data to third party members.
Materials
Tuning Up For A Breakdown
Biomaterials: Acid-sensitive polymers permit tight control over degradation for in vivo drug delivery
by Stephen K. Ritter
December 6, 2010
| A version of this story appeared in
Volume 88, Issue 49

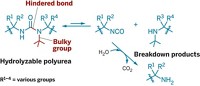
A scientific team based in North Carolina has used tunable silyl ether protecting groups to create a new class of bioabsorbable polymeric materials. These materials can be precisely programmed to degrade by hydrolysis in hours, days, weeks, or months under the acidic conditions found in target tissues, depending on the bulk of alkyl substituents on the silyl ether. This development lays the foundation for a new generation of biodegradable materials that can be shaped into drug-eluting particles, sutures, and coronary stents for medical applications.
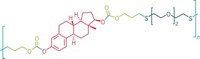
Silyl ethers are among the most popular protecting groups in organic synthesis because the rate of deprotection can be modulated by varying the size of the alkyl substituents on silicon. For example, a simple switch from methyl to tert-butyl groups alters the rate of hydrolytic deprotection by orders of magnitude. Matthew C. Parrott, Joseph M. DeSimone, and coworkers at the University of North Carolina, Chapel Hill, and North Carolina State University applied this chemistry to design easy-to-make materials that are nontoxic and controllably degrade under the acidic conditions in tumor tissue, inflammatory tissue, and diseased cells (J. Am. Chem. Soc., DOI: 10.1021/ja108568g).
The researchers used variously substituted silyl ethers as cross-linkers—in which the silyl group links two acrylate groups—to make different-shaped particles by a process developed earlier in DeSimone’s labs. When internalized in cells, the particles containing methyl-substituted silyl ether linkers degraded in a few hours; those containing the ethyl version showed minimal changes, and those with the isopropyl and tert-butyl versions remained unaltered during the same period. The researchers also made sutures and stents that showed similar patterns of degradation.
A key attribute of the new materials is their ability to resist enzymatic degradation, notes chemical engineer Mark E. Davis of California Institute of Technology. For example, in addition to acid hydrolysis, natural esterases break down polymeric biomaterials that contain ester bonds, like those in the silyl ether-based materials. This can be a problem in vivo, Davis says, because the esterases cause unwanted variations in the biomaterials’ degradation rate. Hydrolysis rates can be controlled by knowing the pH in cells or tissues, he explains, but esterase activity varies from test animals to people and individually from person to person.
“DeSimone’s group has come up with a clever idea in using the silyl ethers, which should shut down enzyme degradation to give highly controlled acid hydrolysis degradation rates, because there are no known esterases that degrade the silicon-based polymers,” Davis says.
The silyl ether technology has been licensed to Liquidia Technologies, a company spun off from DeSimone’s labs a few years ago. DeSimone says Liquidia is expected to use the new bioabsorbable materials to fabricate “Trojan horse” particles to deliver chemotherapy agents and vaccines, as well as drug-eluting coronary stents.



Join the conversation
Contact the reporter
Submit a Letter to the Editor for publication
Engage with us on Twitter