Advertisement
Grab your lab coat. Let's get started
Welcome!
Welcome!
Create an account below to get 6 C&EN articles per month, receive newsletters and more - all free.
It seems this is your first time logging in online. Please enter the following information to continue.
As an ACS member you automatically get access to this site. All we need is few more details to create your reading experience.
Not you? Sign in with a different account.
Not you? Sign in with a different account.
ERROR 1
ERROR 1
ERROR 2
ERROR 2
ERROR 2
ERROR 2
ERROR 2
Password and Confirm password must match.
If you have an ACS member number, please enter it here so we can link this account to your membership. (optional)
ERROR 2
ACS values your privacy. By submitting your information, you are gaining access to C&EN and subscribing to our weekly newsletter. We use the information you provide to make your reading experience better, and we will never sell your data to third party members.
Synthesis
Model Self-Assembly
Molecules that self-assemble form 2-D networks through a discrete series of intermediate stages
by Jyllian N. Kemsley
December 13, 2010
| A version of this story appeared in
Volume 88, Issue 50
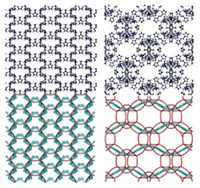
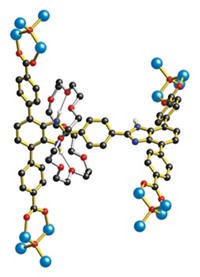
Molecules that self-assemble form two-dimensional networks through a discrete series of intermediate stages, a research group from the University of Strasbourg, in France, has determined (J. Am. Chem. Soc., DOI: 10.1021/ja107882e). Carlos-Andres Palma, Paolo Samorì, and Marco Cecchini used a computer model to simulate the crystallization of melamine and bis(N 1-hexyluracil) into a network of hexagons on a graphite surface to try to pinpoint effects that could aid rational design of 2-D and 3-D structures. In the system studied, the uracil moiety serves as a linker that is hydrogen-bonded to melamine molecules at the network’s vertices. As the network assembles, the molecules transition from a melted phase through a polymer stage and then to a network of various polygons before settling into thermodynamically stable hexagons. Because self-recognition by the molecules can frustrate the process, the researchers propose that using compounds that cannot hydrogen bond to themselves would enhance both the stability and the self-healing ability of supramolecular structures. The computational approach should help researchers compare the chemical nature of structural building blocks and the properties of the resulting architectures, the researchers say.



Join the conversation
Contact the reporter
Submit a Letter to the Editor for publication
Engage with us on Twitter