Advertisement
Grab your lab coat. Let's get started
Welcome!
Welcome!
Create an account below to get 6 C&EN articles per month, receive newsletters and more - all free.
It seems this is your first time logging in online. Please enter the following information to continue.
As an ACS member you automatically get access to this site. All we need is few more details to create your reading experience.
Not you? Sign in with a different account.
Not you? Sign in with a different account.
ERROR 1
ERROR 1
ERROR 2
ERROR 2
ERROR 2
ERROR 2
ERROR 2
Password and Confirm password must match.
If you have an ACS member number, please enter it here so we can link this account to your membership. (optional)
ERROR 2
ACS values your privacy. By submitting your information, you are gaining access to C&EN and subscribing to our weekly newsletter. We use the information you provide to make your reading experience better, and we will never sell your data to third party members.
Biological Chemistry
Back To The Future With Stem Cells
Reprogramming the fate of cells with small molecules
by Sarah Everts
February 8, 2010
| A version of this story appeared in
Volume 88, Issue 6
Of all the obstacles faced by researchers trying to develop stem cell therapies, finding a noncontroversial supply of these omnipotent cells was, for many years, a particularly thorny problem. Opposition, on ethical grounds, to using embryos as a source of pluripotent stem cells—which can morph into any cell of the human body—was so intense that it surely challenged the optimism of even the most ardent advocates of stem cell therapeutics.
But in 2006, researchers delivered a solution to the ethical dilemma. That’s when researchers led by Shinya Yamanaka of Kyoto University figured out how to coax a humble skin cell to become a pluripotent stem cell, instead of destroying an embryo to achieve the same result (Cell 2006, 126, 663). This milestone in cellular alchemy was achieved through genetic engineering: by infecting the skin cell with a virus containing four genes. Expression of these four genes ignited a cascade of signals that morphed the skin cell into one with superpowers in about a fortnight.
Some scientists are trying to reproduce this cellular alchemy with small molecules because gene therapies have been plagued by safety concerns on their way to the clinic. Because small molecules that push embryonic stem cells to differentiate into mature cell types had already been found, researchers began to look for chemical ways to go backward—to reprogram mature cells into so-called induced pluripotent stem cells by using small molecules instead of genetic engineering.
Nowadays, researchers continue to hunt for molecules that can push cells forward and backward in development, while others try to morph one cell directly into other cell types—for example, from a skin cell directly into a dopaminergic neuron that might treat Parkinson’s disease—without the stem cell middleman. The hope is that one day any cell of the body may be reprogrammed into another by means of a simple molecular kit.
Turning one specific cell type into another is plausible in principle but hard to do in practice. Because all the cells in an individual’s body possess the same DNA blueprint, the specific instructions for being a neuron, skin cell, or pluripotent stem cell are all stored in every cell type. Genes unrelated to a specific cell’s modus operandi are put into deep storage by installation of chemical or “epigenetic” marks that prevent the machinery that might translate those unneeded genes from accessing the necessary blueprint.
With such epigenetic marks under the tight control of several hundred enzymes, the real work of reprogramming a skin cell to become a pluripotent stem cell is achieved by directing these enzymes to do a little gene redecoration.
Scientists are still trying to determine the connection between the epigenetic enzymes that do the reprogramming chemistry and the four genes that Yamanaka used to reprogram skin cells into induced pluripotent stem cells. Yamanaka’s genes are blueprints for proteins called transcription factors. These transcription factors control the expression of many genes involved in important cell signaling pathways that are not obviously linked to epigenetic machinery.
Reprogramming biology may still need to be ironed out, but chemical biologists are busy looking for a chemical route to induced pluripotent stem cells. A chemical route is preferable to the four-gene approach for two reasons. The first problem, explains Konrad Hochedlinger, a biologist at the Harvard Stem Cell Institute, is “if you introduce a gene, it needs to integrate into the DNA.” Scientists don’t have control over where precisely in the genome the viruses insert the four genes. It could be inserted smack dab in the middle of another important gene, so that “there’s a danger of mutagenesis,” he adds. The second problem is that two of the four reprogramming genes have been associated with cancer. If reprogrammed stem cells ever make it to the clinic, doctors wouldn’t want to transplant such cells into the pancreases of diabetics, or the spinal cords of paralyzed people, or the brains of Parkinson’s disease sufferers, to cure the disease—and then cause cancer in the same patients.
Although small molecules can cause unwanted side effects, they “are more in our comfort zone in terms of clinical therapies,” say James Chen, a chemical biologist at Stanford University School of Medicine. “Chemists can synthesize and derivatize them, there are standard methods for determining compound pharmacokinetics, and the path to FDA [Food & Drug Administration] approval is well established.” Another benefit of small molecules is that researchers have more control over dosage and delivery time with them than they do with genetic techniques, says Sheng Ding, a chemical biologist at Scripps Research Institute.
In 2007, Ding reported the first small molecule that could stand in for one of the four reprogramming transcription-factor genes. Researchers continue to identify small molecules that can replace one, two, or three of the four reprogramming factors. Among the newest transcription-factor gene stand-ins are molecules such as the lactam kenpaullone from Peter Schultz’s laboratory at Scripps and the heterocycle RepSox from Lee Rubin and Doug Melton at Harvard Stem Cell Institute (Proc. Nat. Acad. Sci. USA 2009, 106, 8912; Cell Stem Cell 2009, 5, 491).
Nevertheless, reprogramming can’t be achieved with only a cocktail of small molecules, Ding says. “At least, not yet.”
Meanwhile, a third reprogramming strategy has emerged, based on just providing to cells the proteins encoded by the reprogramming genes.
Last year, Ding and his German collaborator Hans Schöler at the Max Planck Institute for Molecular Biomedicine, in Germany, demonstrated the strategy. First they coaxed the microbe Escherichia coli into making recombinant versions of the four proteins, each with a few extra arginine amino acids at the end. The arginine served to hijack transport machinery so that the proteins could cross the cell’s plasma membrane, enter mouse skin cells, and jump-start reprogramming into an induced pluripotent stem cell. The downside was that the reprogramming efficiency was much lower than that seen with the use of viruses to install the genes, which was low to start with, he adds. For example, in the best-case scenario, with a virus introducing all four genes into skin cells, reprogramming is successful in only one in a thousand cells over the course of two weeks.
Whatever method researchers use to push cells into more primitive states, the cells must then be redirected toward mature cell types to be therapeutically useful. By screening chemical libraries, researchers have discovered dozens of small molecules that can cause differentiation into a variety of brain, pancreas, bone, and heart cells. But with hundreds of cell types in the body, many more molecules have to be found before scientists possess the power to produce differentiated cells for any occasion and for any animal—what works in mice doesn’t always transfer to humans.
Meanwhile, some researchers are looking for shortcuts that “sidestep the whole business of stem cells,” so that a cell goes directly from one mature cell type to another, explains Stuart Schreiber of the Broad Institute of Harvard University and Massachusetts Institute of Technology. This so-called transdifferentiation was first observed in 1991 when Harold Weintraub, then at the Fred Hutchinson Cancer Research Center, in Seattle, discovered that inserting a single gene into various cell types could turn them into muscle cells. So far, genetic engineering has been the tool of choice for transdifferentiation. For example, by inserting genes, so-called alpha pancreatic cells have been converted into pancreatic beta cells. But several small molecules have also achieved transdifferentiation feats; for example, dexamethasone can convert pancreatic beta cells into liver cells.
Another emerging strategy to manipulate cell fate is to skip the step of culturing cells at all. A great deal of stem cell research aims to develop a population of cells that can be transplanted therapeutically—say beta cells to someone suffering from type 1 diabetes. But others are investigating the possibility of directly reprogramming a patient’s own “endogenous stem cells in vivo instead of transplanting other cells,” says Judith Kimble, a stem cell researcher at the University of Wisconsin, Madison. “Our bodies are chock-full of stem cells. We’ve just got to learn how to manipulate them.”
An existing treatment for cancer already tweaks endogenous populations of stem cells, Kimble points out. In acute promyelocytic leukemia, patients have overproliferation of immature blood cells, which then become cancerous. Retinoic acid can induce the differentiation of these immature cells into a different, non-cancer-causing cell type. “This molecule pushes endogenous, immature cells through from the intermediate proliferating state into a fully differentiated state,” Kimble says.
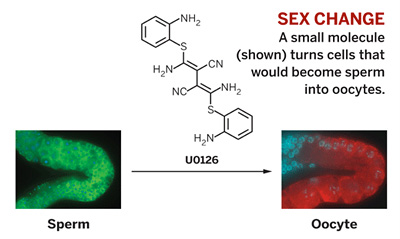
Last fall, Kimble took the idea of tweaking endogenous stem cells a step further: Her research team altered the destiny of worm cells that were fated to become sperm cells by converting them into a population of female oocytes. They accomplished this feat in a living Caenorhabditis elegans worm, using just one molecule (U0126, a butanedinitrile) that the team bought commercially (Nat. Chem. Biol. 2009, 282, 1).
As chemists start putting together small-molecule tool kits for those who wish to tinker with cell fate, they face challenges getting total acceptance from the stem cell community. “Chemists need to really hash out the biology,” Stanford’s Chen says. “It’s not enough to say, ‘I have this compound, and it causes these cells to reprogram in this way.’ Chemists need to get into the trenches with the biologists and figure out why their molecules cause reprogramming or differentiation. And if it doesn’t work with some cell types, they need to figure out why. As chemists, we have to apply the same biological rigor to ourselves that the biologists require from their own community, in order to get them to buy in to the chemical approach.”





Join the conversation
Contact the reporter
Submit a Letter to the Editor for publication
Engage with us on Twitter