Advertisement
Grab your lab coat. Let's get started
Welcome!
Welcome!
Create an account below to get 6 C&EN articles per month, receive newsletters and more - all free.
It seems this is your first time logging in online. Please enter the following information to continue.
As an ACS member you automatically get access to this site. All we need is few more details to create your reading experience.
Not you? Sign in with a different account.
Not you? Sign in with a different account.
ERROR 1
ERROR 1
ERROR 2
ERROR 2
ERROR 2
ERROR 2
ERROR 2
Password and Confirm password must match.
If you have an ACS member number, please enter it here so we can link this account to your membership. (optional)
ERROR 2
ACS values your privacy. By submitting your information, you are gaining access to C&EN and subscribing to our weekly newsletter. We use the information you provide to make your reading experience better, and we will never sell your data to third party members.
Synthesis
Precise Route To Polypropylenes
Polymer Chemistry: Novel chain-transfer process expands range of possible materials
by Stephen K. Ritter
February 15, 2010
| A version of this story appeared in
Volume 88, Issue 7
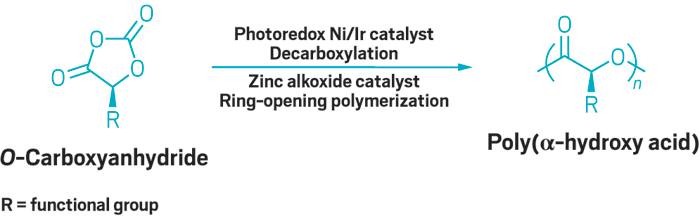
A new strategy for producing propylene- and longer α-olefin-based polymeric materials promises to boost efficiency of polymer synthesis and produce materials with highly controlled polyolefin chain lengths and properties. The process, developed by Lawrence R. Sita and coworkers of the University of Maryland, gives chemists access to previously unattainable oils and waxes that could be useful in specialty chemical applications, including detergents, lubricants, and plasticizers.
“While millions of tons of linear α-olefins are produced each year based on ethylene, the corresponding and potentially more interesting derivatives based on propylene or higher α-olefins have not been prepared in a robust fashion,” says polymer scientist Craig J. Hawker of the University of California, Santa Barbara. “The present work circumvents previous challenges and delivers a low-temperature, highly controlled process that has great potential in both academic and industrial laboratories.”

Sita’s group relied on two existing polymerization techniques to develop the new strategy. The first is living Ziegler-Natta polymerization of α-olefins, which typically involves continuous growth of a single polymer chain on each transition-metal catalyst molecule. The second technique, chain-transfer polymerization, relies on transferring the polymer chain to an inactive main-group metal alkyl, such as diethylzinc, to terminate growth of one chain and initiate growth of a new one. Polymer chemists have recently figured out how to make the chain transfer reversible, thereby opening new avenues to polymer and copolymer production.
Building on the reversible-chain-transfer concept, Sita, Jia Wei, and Wei Zhang use aluminum and zinc reagents in combination with a hafnium catalyst that produces polypropylene as a work-around solution to the one-chain-per-metal restriction of living polymerization (Angew. Chem. Int. Ed., DOI: 10.1002/anie.200906658). “All three metals must act in concert to pull off the precise chemistry,” Sita says.
During polymerization, propene monomer units insert between hafnium and the existing polypropylene chain, Sita explains. The growing chain exchanges rapidly with an oligomer chain residing on the aluminum surrogate by way of the zinc reagent.
The rates of Hf–Zn/Zn–Al transfer are much faster than direct Hf–Al exchange, and all the chain-transfer rates are faster than the propene insertion step, Sita notes. These properties allow multiple propylene oligomer chains to grow at the same rate, giving exquisite control over low molecular weights and narrow molecular weight distributions.
The nature of the chemistry allows preparation of block copolymers and end-group functionalization of oligomers, he adds, such as making long-chain, highly branched alcohols. Sita believes strongly enough in the prospects for the new chemistry that he has helped establish a company, Precision Polyolefins, to commercialize the technology.



Join the conversation
Contact the reporter
Submit a Letter to the Editor for publication
Engage with us on Twitter