Advertisement
Grab your lab coat. Let's get started
Welcome!
Welcome!
Create an account below to get 6 C&EN articles per month, receive newsletters and more - all free.
It seems this is your first time logging in online. Please enter the following information to continue.
As an ACS member you automatically get access to this site. All we need is few more details to create your reading experience.
Not you? Sign in with a different account.
Not you? Sign in with a different account.
ERROR 1
ERROR 1
ERROR 2
ERROR 2
ERROR 2
ERROR 2
ERROR 2
Password and Confirm password must match.
If you have an ACS member number, please enter it here so we can link this account to your membership. (optional)
ERROR 2
ACS values your privacy. By submitting your information, you are gaining access to C&EN and subscribing to our weekly newsletter. We use the information you provide to make your reading experience better, and we will never sell your data to third party members.
Physical Chemistry
Carbon-Carbon Bonds That Are Long And Strong
Coupled diamondoids can take temperatures in excess of 200 °C without decomposing
by Bethany Halford
September 19, 2011
| A version of this story appeared in
Volume 89, Issue 38
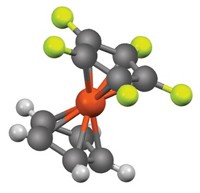
A record has been set for the longest carbon-carbon bond in an alkane. A team led by Peter R. Schreiner of Germany’s Justus Liebig University, Giessen, and Andrey A. Fokin of Ukraine’s Kiev Polytechnic Institute report they have made an alkane with a C–C bond that’s 1.704 Å long (Nature, DOI: 10.1038/nature10367). Typical alkane C–C bonds measure 1.54 Å. The extra-long bond links two diamondoid structures (shown) and exhibits considerable strength, despite its length. Noticeable decomposition of the material occurs only at temperatures above 200 °C. Schreiner, Fokin, and coworkers attribute this stability to intramolecular attractive dispersion interactions—also known as London forces—between hydrogen atoms on the surface of the diamondoids near the elongated C–C bond. They used quantum chemical computations to show that this is the case. “Our findings have consequences for understanding rotational barriers and thermodynamic preferences of branched alkanes over linear alkanes and for the design of structures using attractive dispersion interactions,” the researchers note.


Join the conversation
Contact the reporter
Submit a Letter to the Editor for publication
Engage with us on Twitter