Advertisement
Grab your lab coat. Let's get started
Welcome!
Welcome!
Create an account below to get 6 C&EN articles per month, receive newsletters and more - all free.
It seems this is your first time logging in online. Please enter the following information to continue.
As an ACS member you automatically get access to this site. All we need is few more details to create your reading experience.
Not you? Sign in with a different account.
Not you? Sign in with a different account.
ERROR 1
ERROR 1
ERROR 2
ERROR 2
ERROR 2
ERROR 2
ERROR 2
Password and Confirm password must match.
If you have an ACS member number, please enter it here so we can link this account to your membership. (optional)
ERROR 2
ACS values your privacy. By submitting your information, you are gaining access to C&EN and subscribing to our weekly newsletter. We use the information you provide to make your reading experience better, and we will never sell your data to third party members.
Biological Chemistry
Detecting H2S In Vivo
Studies could lead to sensitive and selective analyses for tiny signaling agent
by Stu Borman
October 17, 2011
| A version of this story appeared in
Volume 89, Issue 42

Despite high hurdles, scientists are making significant progress in developing ways to better measure concentrations of a tiny molecule that could prove as important as nitric oxide in helping to control cellular activities in living organisms.
Like NO, hydrogen sulfide plays an important role in cell signaling. H2S mediates a number of biological processes, including blood pressure, metabolic rate, angiogenesis, and anti-inflammatory effects. Sulfide levels are also altered in conditions like Alzheimer’s disease and diabetes. The ability to measure these levels could be useful diagnostically.
Researchers would love to learn more about how H2S works in the body. But that task has proven difficult because basic tools to measure H2S concentrations selectively and sensitively in tissue and blood, ideally in real time, have been lacking.
It’s difficult to develop probe molecules that are selective for in vivo H2S signaling because cells and tissues contain thiols, sulfenic acids, and sulfites that can compete with H2S for the probes. Also, probes can react with biological compounds to release sulfide, “making it hard to say whether one is measuring sulfide that was there before the probe was introduced,” says cell biologist Mark Roth of Fred Hutchinson Cancer Research Center, in Seattle. Roth’s group has used H2S to temporarily slow metabolism and thereby induce a state of hibernation, which could be useful in treating hypothermia.
Because an effective analytical method for detecting H2S in vivo doesn’t exist, researchers interested in cell signaling have not yet figured out what concentrations of the compound are physiologically relevant. “My guess is cellular H2S concentrations are submicromolar—maybe nanomolar or less,” says physiologist Kenneth R. Olson of Indiana University School of Medicine, who specializes in blood pressure and biological-fluid regulation. But H2S concentrations spanning five orders of magnitude have been reported in the scientific literature.
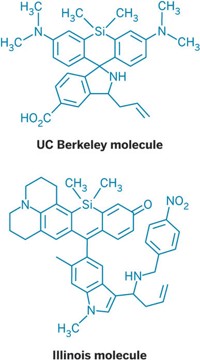
Several research teams are trying to improve the situation. For example, this summer, a group led by chemist Christopher J. Chang of the University of California, Berkeley, developed the first approach for detecting H2S in living cells.
Their system uses a pair of fluorescent probes, which they call Sulfidefluor-1 and -2. The probes have azide groups that are reduced by H2S to form fluorescent amines. The probes are highly selective for H2S over other “biologically relevant reactive sulfur, oxygen, and nitrogen species,” the researchers note in a recent paper (J. Am. Chem. Soc., DOI: 10.1021/ja203661j). New versions of the probes, developed since the paper was written, “are already revealing endogenous H2S bursts in a variety of cellular models,” Chang tells C&EN.
Neuroscientist Solomon H. Snyder of Johns Hopkins University says his team is about to begin collaborating with Chang “to evaluate his assay, especially utilizing the new, unpublished, more-sensitive probes.” The assay “certainly detects H2S with sensitivity and specificity. We are interested in monitoring basal, physiologic levels of H2S and don’t know whether the published assay has adequate sensitivity.” Nevertheless, he says, “the published assay and, especially, improved versions with the more sensitive probes may well markedly augment the ability of investigators to assess physiologic roles for H2S.”
Chemist Chuan He of the University of Chicago and coworkers recently developed another in vivo H2S detection system (Nat. Commun., DOI: 10.1038/ncomms1506). Their fast and sensitive probes, which they call SFP-1 and -2, react selectively with sulfides in vivo, even in the presence of high background concentrations of thiols. The probes’ aldehyde and adjacent unsaturated acrylate ester selectively capture sulfide, forming thioacetals that fluoresce.
Higher sensitivity, faster response, and further confirmation of validity and in vivo sulfide selectivity are probably needed before the Chang and He groups’ techniques can become generally useful. Until the techniques are further verified and improved, “the fundamental detection problem remains,” Roth says. Nevertheless, “both groups have done excellent work,” he adds.
Making it possible to measure H2S in vivo is the two techniques’ “biggest contribution,” Olson says. “I don’t think either of them do it well enough yet. The reaction time is quite long”—minutes to hours, as opposed to real time. “And I’m not sure they have enough sensitivity. So I don’t think either one of these is usable right now to give us good physiological information. But they’re fantastic in directing the path of where to go in the future.”


Join the conversation
Contact the reporter
Submit a Letter to the Editor for publication
Engage with us on Twitter